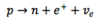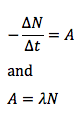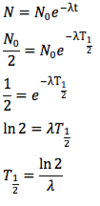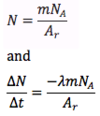Topic 13 - Quantum physics and nuclear physics Flashcards
(40 cards)
What is the photoelectric effect?
When electromagnetic radiation is directed onto a clean surface of some metals, electrons are ejected.
What are photoelectrons?
Electrons ejected due to the photoelectric effect from the surface of some metals
What kind of EM waves can produce the photoelectric effect?
- Under suitable conditions, visible light, X-rays, and gamma rays
- Most often ultraviolet radiation is used together with a zinc plate
What are the key features of the photoelectric effect?
- If the intensity of the radiation is increased more photoelectrons are being released every second
- There is no time delay between the radiation reaching the metal surface and the emission of photoelectrons → release is instantaneous
- The effect can only occur if the frequency of the radiation reaches a certain minimum value known as the threshold frequency, f0
- For a given incident frequency the effect occurs with some metals but not with others (due to different f0 of metals)
What is a photon?
A packet of energy in electromagnetic radiation (instead of continuous waves)
What is the energy carried by each photon?
E = hf
where
h = Planck’s constant,
f = frequency of the radiation
Since c = fλ:

Why can’t the wave model of light be used to explain the photoelectric effect?
Because
- There would be a delay before the effect begins after the wave hits the surface
- Radiation of any frequency would cause the photoelectric effect if the intensity is high enough
Both are wrong
Outline the Einstein model of photoelectric effect
- Explains the effect using the concept of photons
- When a photon interacts with an electron, it transfers all of its energy to that electron
- A single photon can only interact with a single electron
- Increasing the intensity of radiation only increases the no. of photoelectrons, not their energies
- Some of the energy of the photon is used to overcome the attractive forces of the electron
- The remaining energy is transferred to the kinetic energy of the photoelectron
What is work function, ϕ?
- The energy required to remove different electrons is not always the same
- There is a minimum amount of energy needed to remove an electron, called the work function
- Different metals have different values for their work function
What is the relationship between a photon’s energy, hf, and the work function, ϕ, of a metal?
hf < ϕ
- Less energy than the work function
- The photoelectric effect cannot occur
hf0 = ϕ
- Exactly the same energy as the work function
- The effect occurs and a photoelectron is released, but the electron will have zero kinetic energy
hf > ϕ
- More energy than the work function
- The effect occurs
What is Einstein’s photoelectric equation?
hf = ϕ + Emax
hf = hf0 + Emax
where
hf = energy carried by photon,
ϕ = work function,
Emax = maximum kinetic energy of photoelectron
hf0 = ϕ
What is the stopping potential, Vs, in the Einstein model?
The potential on the anode needed to just stop all photoelectrons reaching it
Emax = eVs
How can Einstein’s photoelectric equation be re-written with the help of stopping potential?
hf = ϕ + eVs
or
hf = hf0 + eVs
What is the de Broglie hypothesis?
All moving particles have a wave-like nature. The wavelength of a moving particle is related to its momentum:
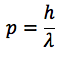
What is meant by matter waves?
Matter can also behave like a wave (photons and electrons)
How did the Davisson-Germer experiment prove de Broglie’s hypothesis?
- Since electrons are particles that behave like waves, they should also be diffracted like light
boom
- They were diffracted
How can the emission spectrum of an atom be investigated?
Atoms emit electromagnetic radiation when excited by heating or passing an electric current through its gas
- A spectrometer is used
- A beam of light from excited gaseous atoms is passed through a lens via a slit to a second lens and then to a prism
- The prism separates the spectral lines, which can be observed and measured using an eyepiece
How can the absorption spectrum of an atom be investigated?
- White light is shone through a sample of a gaseous element (at low pressure and temperature)
- The light that emerges has some wavelengths missing
- These bands in the spectrum are the element’s characteristic wavelengths
- The position of these bands is the same as the position of the lines in teh emisison spectrum
Explain how energy is quantized in atoms
- Electrons can only exist in certain energy levels
- They behave like stationary waves fitted into the circumference of the orbit
- A free electron at n = ∞ has zero energy
- n = 1 has the lowest energy
- The values are negative because energy is lost as the electron moves closer to the nucleus
- For an electron to move between energy levels, energy must be absorbed or given out
- Thermal energy raises some electrons to an excited state
- An excited electron will quickly fall back to a lower energy level

How do atomic spectra provide proof for the quantization of energy in atoms?
- When electrons fall from their excited state, the downward transition corresponds to the emission of a photon whose energy is the same as the energy difference between the energy levels:
hf = E2 – E1
- Similarly, photons can only be absorbed if their energy corresponds to the energy difference between two energy levels
- Further away from the nucleus, the energy levels become closer and closer and show convergence of lines at high frequency
How is the electron in a box model used to rationalise the atomic energy levels?
- The model uses the idea of the electron behaving as a wave to explain why the energy levels in atoms are quantized
- Electorn is confined in a small region between two walls
- If the electron is a wave, it must show boundary conditions (standing wave)
- The wave has nodes and antinodes
- The walls of the box must always be nodes
- The waves must have a whole number of half wavelengths within the box
- The wavelength of the electron wave is determined by the size of the box
How is the wavelength and energy of an electron given by the electron in a box model?
me = mass of electron
n = the integer in the box (idk?)
L = length of the box
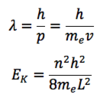
What is the Schrödinger model of the hydrogen atom?
- The model assumes that electrons in the atom may be described by wavefunctions, Ψ(x, t)
- The electron has an undefined position
- The square of the absolute value of the amplitude is a measure of the electron density (probability distribution) in a region of space:
|Ψ(x, t)|2

What does the Heisenberg uncertainty principle suggest?
That it is impossible to know both the momentum and the displacement at the same time: the more accurately we know the speed of the electron (the smaller the ∆p), the less we know about where it is (the larger the ∆x)
Relationship can be written as: