Thyroid Flashcards
(131 cards)
Shape and location of thyroid
Butterfly shape and located below larynx
Blood supply of thyroid
large blood supply, like all endocrine organs
Which kind of nerve innervate thyroid gland?
Innervated by sympathetic nerves
What is the functional unit of thyroid gland
thyroid follicles
What are thyroid follicles made of?
consisting of a single layer of epithelial cells surrounding a lumen that contains colloid
WhaT is colloid made of
contains the prohormone thyroglobulin
What are parafollicular cells? What is their role?
Parafollicular cells, also called C cells, are neuroendocrine cells in the thyroid.
The primary function of these cells is to secrete calcitonin. T
hey are located adjacent to the thyroid follicles and reside in the connective tissue.
What is the function of thyroid hormone?
to secrete the quantity of thyroid hormone to meet the demand of peripheral tissues
each follicle is marked by a __
each follicle is marked by a basement membrane
Difference between inactive and active epithelium lining the folicular cells?
inactive epithelial cells are cuboid active-> columnar
What regulates the thyroid hormone release? How?
The blood flow regulates the thyroid hormones release by affecting the the delivery of TSH, iodine and nutrients
Where are cilia found in thyroid follicular cells
present towards the epical end of the cells, towards the lumen where colloid is
__nerves control the blood flow through the gland
What does this control in effect?
Postganglionic sympathetic nerves control the blood flow through the gland
thus determines iodine and TSH supply, determining T3 and T4 synthesis
Overactive vs inderactive throid gland cells

What are the biologically active forms of thyroid hormones? WHat are the inactive forms?
T4 and T3- active
inactive: rT3 and T2
Wher are rT3 and T2 formed?
in the periheral tissues
Which percursors of thyroid hormones do not leave the cells?
Monoidootyrosine
Diiodotyrosine
Chemcial names of T4 and T3? How are they assigned?
the one ring closest to COOH amino terminal is numbered witout primes
the furthest ring is given a prime

What kind of receptor is used by TSH? Which pathways?
GPCR receptor- cAMP or IP3/DAG
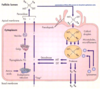
How does iodine get into our bodies?
- Epical side of intestinal cells have cilia
- iodine has to come from the diet and enter the epithelial cells
- PDS is present on the epical side; a transporter of iodine that moves it into the collide
- iodine is charged- is brought into the blood by a transporter NIS (sodium iodide symporter) -co-transporter; ions are transporter in the same direction
- iodine levels in the blood are much lower than the levels in epithelial cells or colloid-> iodine is transported by active transport against the gradient
- Sodium is brought down the gradient, iodine is brought against the gradient
- Sodium-potassiumATPase- maintains sodium gradient across cell membrane; present in all cell; results in less sodium in the cell compared to the outside

Describe iodine synthesis pathway
1) Iodine, as it is charged, is transported from plasma into follicular cells across the basal lateral membrane via Na-I symporter (NIS); uses electro-chemical gradient to drive the transport of 1 iodine ion and 2 Na in the same direction
2) Na is then transported out of the cell back to the blood via K-Na ATPase;
via the iodine ion is transported across the epical membrane into the colloid space via PDS that is present on the epical side; a transporter of iodine that moves it into the collide
Thyroglobulin is protein that comes to colloid where now thyroglobulin has to be iodinated
iodination requires hydrogen peroxide
Dual oxidase (DUOX) creates H2O2-> thyroglobulin is now iodinated
Iodinated thyroglobulin can have mono or diiodo tyrosine; It also has T3 and T4;
Iodinated thryglobulin is internalized => mono or diiodo tyrosine are cleaved off and are deiodinated; this iodine that comes from mono and diiodo tyrsines are recyceld
they come back to the pool of iodine in colloid; tyrosine gets recycled for the synthesis of thyroglobulin
T3 and T4 are now created and are transported out but we don’t know how
Iodination of tyrosine molecules causes thyroid peroxidase to conjugate neighbouring tyrosine residues, forming either T3, T4or rT3
TG protein which is now iodinated will be endocytosed
Lysosomes containing proteases will fuse with TG protein containing versicle and cleave peptide bonds where T4. T3 or rT3 are located
Once cleaved T4 and T3 are transported out of the cell

What is “trapping” in the process of T4/T3 synthesis
active transport of iodine into the thyroid cell via Na-I symporter
lots of iodine is required- at least 3 iodines per hormone-> iodine is trapped
What is “organifaction” in the process of T4/T3 synthesis
oxidation of iodide and iodination of tyrosyl residues in thyroglobulin
iodine cannot stay in its organic form as it would contribute to the charge
becomes organic as it is present in an organic molecule
What is known as “coupling” in the process fo T4/T3 production
linking pairs of iodotyrosines in thyroglobulin to form T3 and T4