Theme 2- core clinical immunology- week 1 Flashcards
What is the atopic triad?
Triad- rhinitis, dermatitis, asthma
What does asthma cause?
Airway inflammation
What is rhinitis?
Rhinitis, also known as coryza, is irritation and inflammation of the mucous membrane inside the nose.
What does rhinitis cause?
Blocked / runny / itchy nose, sneezing - often with eye symptoms (Itching / burning / watery eyes, redness)
What does rhinitis lead to?
Leads to allergic and non-allergic subdivisions
What does the allergic response to rhinitis lead to?
Allergic leads to seasonal and perennial:
Seasonal- pollen and moulds
Perennial- house dust mite, animal dander
What does the non-allergic response of rhinitis lead to?
Non-allergic leads to vasomotor, infective, structural, drugs, hormonal and polyps
What is the treatment of rhinitis?
Treatment – Antihistamines and intranasal steroids
What is asthma?
Disease of inflammation & hyper-reactivity of small airways
Who does asthma mostly affect?
In childhood - aero-allergic stimuli - house dust mite key pathogenic importance
What is asthma mediated by?
Immediate symptoms are IgE-mediated
What does asthma cause?
Damage to airways due to late phase response
Damaged airways are hyper-reactive to non-allergic stimuli e.g. Fumes
What is the pathogenesis of asthma?
Mechanism- allergen presenting by APC –> T cell proliferation and differentiation into a TH2 cell releasing cytokines- IL4, IL13 and IL5–> B cell plasma cell forming mast cells, basophils and TH2 cell forms eosinophils–>Mediator- histamines, leukotrienes, prostaglandins, cytokines and basic protein and enzymes–> Leads to asthma

What is dermatitis?
CLINICALLY - Intense itching, blistering/weeping, cracking of skin

What is the major trigger in atopic disease of dermatitis?
HOUSE DUST MITE now thought to be MAJOR TRIGGER in atopic disease
What is the treatment for dermatitis?
Topical Steroids & Moisturisers
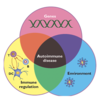
What is atropy?
Atopy refers to the genetic tendency to develop allergic diseases such as allergic rhinitis, asthma and atopic dermatitis (eczema).
What is non-atopic?
Some conditions are not dependent on IgE but still involve an abnormal immune response to a wide variety of external environmental agents. These conditions are known as non-atopic (non-IgE-mediated).
What causes an itch?
Filaggrin protein- main barrier function- allergic reaction leads to distruption and leads to a Th1 response then Th2 response leading to secretion of IgE. Leads to increased IL-31 causing itch.

How does sentisation lead to exposure?
IgE exposure on B cells and APC–> activates T cells- Th1 to TH2 cells via cytokines–> Th2 main allergic response–>sends to B cells to secrete IgE then differentiate into plasma cells to release IgE–> Crosslinking of mast cell and basophil cell-surface –> release of vasoactive amines–> lipid mediators, chemokines and cytokines
Summary of Late Phase Inflammation
- Allergic inflammation (late phase of the allergic reaction).
- Following migration to sites of allergen exposure under the influence of chemokines and other cytokines, allergen-specific T cells are reactivated and clonally expand.
- Local IgE-facilitated antigen presentation by dendritic cells (DCs) increases T-cell activation.
- Local IgE production is seen in allergic rhinitis and asthma but not in allergic skin inflammation (the main example of which is atopic dermatitis).
- Eosinophils are one of the main inflammatory cells
- TH1 cells, which produce interferon- (IFN) and tumour-necrosis factor (TNF), contribute to the activation and apoptosis of keratinocytes (in the skin), bronchial epithelial cells and pulmonary smooth-muscle cells.
- Activation of mast cells and basophils, which release histamine, chemokines and other cytokines
What is specific IgE testing?
An allergen-specific immunoglobulin E (IgE) test measures the levels of different IgE antibodies.
What occurs in specific IgE testing?
Allergen binds to different things add patients serum, antibodies will bind then need to confirm if antibody IgE type, need to bind an antibody that binds to the IgE then need a mechanism for identification which will change colour.
Specific IgE Testing (>0.35 KuA/L)
What is the skin prick test?
Skin prick test (>2mm wheal)- prick the skin so can trigger IgE reaction- will form a wheal over 2mm of the negative control (usually 0mm) for an allergic reaction
What is the intra-dermal skin test?
Intra-dermal test- more invasive- get more allergen than in the skin prick test. Inject tiny amount into the dermis and then mark the size of the circle that has been drawn around the bleb. No control needed. Size need to increase 3mm from site of injection. Takes longer than skin prick test. Primarily done for brochoallegies.
What is the basophil activation test?
The basophil activation test (BAT) is a flow-cytometry-based functional assay that assesses the degree of cell activation after exposure to a stimuli.
How does the basophil activation test work?
Antibody binds to basophil. Then add to add allergen. This triggers the basophils, makers on the basophil increase expression.

What is the graded challenge test?
Graded challenge test- exposure to patient slowly and watch if any reactions
Gold standard test for allergy- graded challenge test
What are the advantages and disadvantages of specific IgE testing?
Specific IgE Testing
Safe
False negatives
False positives
What are the advantages and disadvantages of the skin prick test?
Skin Prick Test
Quick
Patient satisfaction
False negatives
False positives
Antihistamines
Slight risk
Treatment of common allergies?
Symptomatic
Antihistamines, Steroids, Adrenaline
What is specific- immunotherapy (subcutaenous or sublingual- under the tongue) used for?
Indications:
Life threatening reactions to Wasp & Bee sting
Severe Hay fever
Animal dander allergy
Not Helpful:
Multiple allergies
Food allergy
Eczema
Spontaneous Urticaria
What does allergic-specific immunotherapy allow?
Allergen-specific immunotherapy (SIT) improves the quality of life of treated individuals and has been shown to reduce both symptoms of allergy and medication use in controlled clinical trials.
What is the mechanism of SIT?
- reduces mediator release
- reduces number of cytokines in the blood, increased cytokines in tissues
- decrease in allergen specific IgE
- increase in ILs
- prevention of new sensitisation and reduces symptoms of allergic reaction
What are major food allergens?
- Water soluble glycoproteins 10 - 60 kd
- Cow’s milk
- Egg
- Legumes - peanut; soybean; tree nuts
- Fish
- Crustaceans / molluscs
- Cereal grains
What are the adverse reactions to foods?
- Gastrointestinal- vomiting, diarrhoea, oral symptoms
- Respiratory (upper & lower)- rhinitis, bronchospasm
- Cutaneous- urticaria, angioedema, role of food in atopic dermatitis unclear
- Anaphylaxis
Why does someone reaction if they have no IgE detected?
Different sensitivities
When taking someones history for a drug what should you find out?
- Indication for the drug
- Detailed description of the reaction
- Time between drug intake and onset of symptoms
- Number of doses taken before onset
- Aware of pharmacological effects and non-immunological ADR
When managing someones drug allergy what should you do?
Management
- Intradermal testing (maybe contraindicated in some situations)
- Graded challenge
- Desensitization
What cells are seen in the innate immunity?
- Macrophages
- Dendritic cells
- Mast Cells
- Neutrophils
- Complement
What cells are seen in adaptive immunity?
- T cells
- B cells
What is the innate response? Recognition of what? Memory? Regulation? Response time? Duration?
- Pattern recognition against broad classes of antigen
- No memory
- No amplification
- Little regulation
- Fast response (hours – days)
- Short duration
What is the adaptive response? Recognition? Memory? Regulated? Response time? Duration?
- Highly specific (T and B cell receptors)
- Strong memory and amplification component (e.g. vaccines, previous infection)
- Many regulatory mechanisms
- Slow response (days to weeks for initial exposure)
- Responses may last months - years
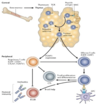
How does the innate response occur?
Innate immune cells directly detect and attack antigenic targets (e.g. microbes) Occurs at sites of infection – e.g. barrier organs
- Phagocytosis
- Cytotoxicity- complement sticks to cell walls and destroys them
- Inflammatory mediators and chemokines to attract other cells
- Dendritic cells present antigen to T cells
- Cross talk between DCs, T cells and B cells
- Immune memory to determine specific learned responses
- Occurs in lymphoid tissues

How does the adaptive immune system respond?
Adaptive immune cells activate innate immune cells, directing tissue inflammation to specific targets
T cell cytokines activate monocytes, macrophages
B cell antibodies activate complement

What are phagocytic cells?
Phagocytic cells
Neutrophils: eat and destroy pathogens
Macrophages: also produce chemokines to attract other immune cells
Dendritic cells: also present antigen to adaptive immune system
What are histamine producing cells?
Histamine-producing cells
Mast cells, basophils, eosinophils: produce histamine and other chemokines and cytokines
Vasodilatation, attract other immune cells
Defence against parasites, wound healing but also allergy and anaphylaxis
What are complements in innate immune system?
Complement
Directly attacks pathogens via alternative and lectin pathways
May be activated by adaptive immune system via antibodies
What are cytokines?
Cytokines
Signal between different immune cells (e.g. innate to adaptive, adaptive to innate)
What are chemokines?
Chemokines
Attract other immune cells to sites of inflammation
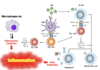
How do T cells cause inflammation by inflammatory cytokines or by helping B cells make autoantibodies?
APC picks up antigen, goes to T cell can presents it (immune synapse- one cell talking to the other), T cells can convert naïve T helper cell to a T helper 1 cell, which makes inflammatory cytokines.
Another thing T helper 2 cells- cross talk with B cells into memory B cells which make plasma cells and produce lots of autoantibodies.
Net results of this is inflammation.
What do autoantibodies do?
Auto antibodies- Some autoantibodies directly interfere with normal physiological function rather than causing inflammation and tissue damage.

Definition of autoimmunity?
Defining characteristic: the adaptive immune system recognises and targets the body’s own molecules, cells and tissues (instead of infectious agents and malignant cells)
How does autoimmunity occur?
Main characteristics:
- T cells that recognise self antigens
- B cells and plasma cells that make autoantibodies
- Inflammation in target cells, tissues and organs is secondary to actions of T cells, B cells and autoantibodies