Testing/Imaging Flashcards
(58 cards)
How can chromosomal abnormalities cause disease?
Altering the concentration of particular gene products
- Duplication or amplification (typically oncogenes)
- Deletion or gene interruption (typically tumor suppressor genes)
Altering the gene product by producing a new fusion gene with altered expression and/or a new fusion protein with altered function.
- Example: fuse regulatory region from one gene with functional portion of another such that expression levels, timing and/or distribution within or among cells is altered.
- Example: Alter the function of a protein by substituting one functional domain with another (e.g. substitute one DNA binding site with another).
CML accounts for ____% of all leukemia and occurs most frequently in ____ year olds.
CML accounts for 15-20% of all leukemia and occurs most frequently in 40-50 year olds.
CML was the first cancer to be associated with a ________.
specific recurrent chromosome abnormality
CML is characterised by a ___ chromosome that had formed as a result of a ___ translocation
CML is characterised by a Philadelphia chromosome that had formed as a result of a 9;22 translocation
The _____ or Philadelphia chromosome contains a fusion gene composed of the ______ serine/threonine kinase gene on 22 and the _____ tyrosine kinase gene on chromosome 9.
The der(22) or Philadelphia chromosome contains a fusion gene composed of the BCR (breakpoint cluster region) serine/threonine kinase gene on 22 and the ABL1 (Abelson1) tyrosine kinase gene on chromosome 9.
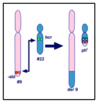
The new fusion BCR/ABL1 tyrosine kinase produced by the fusion gene is permanently _____ and activates a number of ______. (CML)
The new fusion BCR/ABL1 tyrosine kinase produced by the fusion gene is permanently turned on and activates a number of signal pathways. (CML)

Permanent _____ of the BCR/ABL1 fusion kinase leads to _____ by interfering with basic cellular processes.
Permanent activation of the BCR/ABL1 fusion kinase leads to malignant transformation by interfering with basic cellular processes.

95% of patients with CML carry a _____ translocation
Recriprocal 9;22

Explain the probe placement used for BCR-ABL1 sequencing in CML patients:
An orange/yellow probe is placed at the bottom of chromosome 9
A green probe is placed spanning the BCR region of chromosome 22 and upstream on 22 as well.

What would a FISH assay look like for a patient with CML (who has a Philadelphia chromosome)?
Top is normal - see two greens and two reds. Can see in interphase, aka non-dividing cells as well.
In mutant - see red and green signals on normal 9 and 22, and also see a yellowy/white signal on the derivative 9 and derivative 22 as well. See abnormal pattern in interphase, non-dividing cells too.

_____ is the first-line drug of choice for the treatment of most patients with CML in chronic phase. WHY?
Imatinib.

ATP is normally bound to BCR-ABL, resulting in the phosphorylation of a tyrosine on the substrate (cancer protein). The substrate is then able to interact with an effector protein and multiple cancer pathways are stimulated. Right panel, imatinib is bound to BCR-ABL in place of ATP. The tyrosine of the substrate is not phosphorylated, and the substrate can no longer interact with the effector protein.
Because the BCR-ABL1 tyrosine kinase enzyme exists only in cancer cells and not in healthy cells, imatinib works as a form of targeted cancer cells are killed through the drug’s action. Well tolerated and induces long-lasting remissions.
Imatinib is a ____ inhibitor developed to treat ____
tyrosine kinase inhibitor
CML treatment

What is the major cause of relapse in cancer and leukemia?
Minimal residual disease - small numbers of cancer cells that remain in the patient during treatment, or after treatment when the patient is in remission.
How are patients treated with Imatinib monitored post-treatment?
Monitored at 3- 6 month intervals until cytogenetic remission is obtained then yearly to monitor for marrow dysplasia or the emergence of new clones with additional chromosome abnormalities [+8, i(17q), additional copies of der(22)] associated with disease progression. Molecular monitoring is also performed.
In the lab, do this with INTERPHASE FISH. Allows us to look at many cells quickly. Can see zoomed-in verson and see difference in signaling.

How is karyotyping helpful in identifying additional clones that signal disease progression in CML patients?
Allows us to identify additional clones that would signal disease progression.
This patient has remaining 9 cells that carry translocation that also carry an extra 8 and 17. This suggests progression and worsening, with a clone that has more abnormalities.
FISH would have identified the 9;22 translocation, but not the other abnormalities that are signaling/suggesting disease progression.

Chromosome abnormalities involving the MLL (KMT2A) gene have been implicated in at least 10% of _____ (e.g. __ and __)
Chromosome abnormalities involving the MLL (KMT2A) gene have been implicated in at least 10% of acute leukemias (e.g. AML and ALL)
The MLL (KMT2A) gene mutation is located at __ (site) and encodes a transcriptional regulatory factor essential for _____ and self-renewal of ______ and _____ progenitors
The MLL (KMT2A) gene mutation is located at 11q23.3 (site) and encodes a transcriptional regulatory factor essential for embryonic body plan formation and self-renewal of hematopoietic stem cells and immature progenitors
Why is the MLL gene associated with a poor prognosis?
Currently no targeted therapies for patients with MLL rearrangements.
Some exceptions do exist however, such as a deletion or inversion of 11q23
Recurrent ____ translocations are associated with at least 80 partner genes
MLL (KMT2A)
The mechanism whereby the multiple MLL fusion partners initiate cellular transformation is largely unknown.
Fusion protein acts as a dominant gain-of-function mutation (possibly increased multimer formation and/or stability of fusion protein).
Can traditional G-banding be used to identify MLL (KMT2A) rearrangements?
Yes
This patient carries a 4-11 translocation. Fuses the MLL gene to AF4 gene. Look for a short chromosome 4 with a little 11 hanging off it, and a long 11 with a lot of 4 hanging off it.

Given there are multiple pairing partners in patients with the MLL mutation, ____ is used rather than dual fusion FISH.
Break Apart
Use a green probe that hybridizes to 5’ end and upstream sequences, and a red probe that fuses to 3’ end and downstream sequences.
In any case with a MLL rearrangement, there will be a breakpoint in the MLL gene, so the red probe will migrate to new partner location.
PROS: this allows us to detect basically all the MLL rearrangements
CONS: it doesn’t tell us who the partner chromosome is.

Which of the following samples would be most appropriate for chromosome microarray analysis?
A. A bone marrow sample from a patient suspected of having ALL secondary to a t(4;11)(q21;q23)
B. A sample from a patient suspected of having neuroblastoma with amplification of the MYCN gene as well as surrounding genes
C. A follow-up bone marrow sample looking for minimal residual disease in a patient with CLL and the following karyotype at diagnosis: 46,XY,-11,del(11)(q23),+12,del(21)(q22)
B. A sample from a patient suspected of having neuroblastoma with amplification of the MYCN gene as well as surrounding genes
A is balanced, which isn’t detectible
C shows minimal residual disease, so it’s not detectible.
What are limitations of chromosome microarray analysis in cancer patients?
•Can’t detect balanced rearrangements
- No gain or loss present to identify the balanced t(9;22) associated with CML or the t(4;11)(q21;q23) seen in ALL
•Low level mosaicism/clonal evolution
- in setting of minimal residual disease the data from the normal cells would mask the data from the abnormal cells
- minor clones that provide information about disease progression may be difficult to detect
- Abnormalities present in less than 10-15% of cells are typically NOT detected




