Signaling Flashcards
(28 cards)
Two types/locations of receptors
Receptors can be located in the
cytoplasm or in the plasma membrane of the cell.

Membrane receptors:
Large or polar ligands cannot cross the
lipid bilayer. Insulin, for example, is a protein hormone that cannot diffuse through the plasma membrane; instead, it binds to a transmembrane receptor with an extracellular binding domain.
Cytoplasmic receptors:
Small or nonpolar ligands can diffuse
across the nonpolar phospholipid bilayer of the plasma
membrane and enter the cell. Estrogen, for example, is a
lipid-soluble steroid hormone that can easily diffuse across the plasma membrane; it binds to a receptor in the cytoplasm.
Signaling method: ligand-gated channels
Ligand binds to receptor on cell surface
⇓
Open a few ligand-gated channels
⇓
A little ion flow
⇓
Hit threashold voltage
⇓
Open many (voltage gated) channels
⇓
big chainge in ion concentrations
⇓
BIG EFFECT
Signaling example: ligand-gated channels
Acetyl choline (AcCh)
Ligand binds to receptor on cell surface
AcCh binds
⇓
Open a few ligand-gated (Na+) channels
⇓
A little ion* (Na+) * flow
⇓
Hit threashold voltage
less (-) inside
⇓
Open many (voltage gated Na+) channels
⇓
big chainge in ion (Na+) concentrations
⇓
BIG EFFECT
Muscle contracts
Signaling Method: Cascades of Modification
ligand (1st messenger)
⇓
activate receptor in membrane
⇓
activate protein inside cell (usally a chain of activtions = cascade)
⇓
activate a lot of target protein (enzyme or TF…
⇓
lots of product
Examples: Hormones
TSH & epinephrine
- TSH:* stimulates release of thyroid hormone (thyroxine of TH) from thyroid gland.
- Epinepherine* stimulates glycogen breakdown.
*Many water soluble hormones work this way.
Signaling Method: by affecting transcription/translation
Ligand binds
⇓
activate a TF
⇓
transcribe a gene
⇓
make lots of mRNA molecules/gene
⇓
mRNA translated
⇓
many new protein molecules/mRNA
Examples:
Thyroid hormone (thyrotropin or TH) & steroid hormones.
Most lipid soluble hormones act in this way. Receptor itself is a TF.
Lipid Soluble Ligands
- All use intracellular recptors (steroids, TH, retinoids, vit A, vit D)
- cannot be stored (can pass through membranes, so must be made from soluble precusors as needed
Intracellular Receptors
_Types: _
- *lipid soluble ligands *
- hormone binding proteins are needed in blood (are not water soluble, so all lipid soluble ligands travel in blood bound to soluble proteins
All are TFs: activate or repress transcription
- Example: HRE
Have at least 3 domains:
- Transcription activating (or inhibiting) domain
- DNA Binding Domain: binds to HRE (specfic to each hormone)
- Ligand binding domain: binds to a particular steriod (or thyroxine, etc)
- Other domains: receptors also need NLS, and region that allows dimerization, these may be included or separate from the domains listed above.
Usual course when TF receptors bind to lipid soluble ligands: Receptor activtion and transcription effect
- Binding: receptor binds its ligand. Causes conformational change of at least one domain of protein receptor.
- Disassociation: receptors disassociate from inhibitory proteins.
- Dimerazation: pairs formed
-
Location:
•If receptor is in cytoplasm, NLS is uncovered, and receptor moves to nucleus. - DNA binding: activted receptor (dimerized & bound to ligand) binds to HRE on DNA.
- Effect on transcription: activated receptor binds to other proteins associated with the DNA (other TF’s and/or coactivators or inhibitors and stimulates or inhibits transcription)
Why do you get different results (patterns of transcription) for different genes in response to the same lipid soluble hormone?
Different genes have different cis-acting regulatory sites. (different genes respond differently to the same combination of TFs)
NOTE: All cells have the same DNA: therefore
- all cells (except immune system have the same cis-acting regulatory sites: same HREs, enhancers, etc
- TRANS-acting factors (ie: horomone receptors and other TFs that vary, between cells, not the cis-acting regulatory sites
- cis-acting regulatory sites do vary beween GENES
- all cells have the same genes for the trans acting factors, receptors, etc. but different genes are used (expressed) to make diffeent regulatory proteins in different cells.
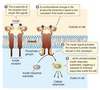
A Protein Kinase Receptor
The mammalian hormone insulin binds to a receptor on the outside surface of the cell and initiates a response.
A Gated Ion Channel
The acetylcholine receptor (AChR) is a
ligand-gated ion channel for sodium ions. It is made up of five polypeptide subunits. When acetylcholine molecules (ACh) bind to two of the subunits, the gate opens and Na+ flows into the cell. This channel helps regulate membrane polarity

GPCR (G Protein Coupled Receptors) Antagonists
block the action of the normal ligand; blocks signaling even in the presence of normal ligand.
GPCR (G Protein Coupled Receptors) Agonists
Mimic the action of the normal ligand, causes signaling in the absence of normal ligand.
The Structure of G Protein-Linked Receptors
Each G protein-linked receptor has seven transmembrane helices.
A ligand binds to the extracellular portion of the receptor, causing an intracellular portion of the receptor to bind and activate a G protein.
Besides the regions shown, the second cytosolic loop is also involved in G protein interactions in some cases.
Specific amino acids in the cytosolic region are also targets for phosphorylation by G protein-linked receptor kinases (GRKs) and protein kinase A.

Compare and contrast G Proteins and GPCRs
G Proteins: activated by receptors
GPCRs: the actual receptor itself
Enzyme linked receptors
- Structure: usually single pass proteins that aggregate into dimers when activated
- Function: Many Growth Factors use TK linked receptors or related receptors
- How? Usually generate cascades of modification, but do not usually use 2nd messengers.
Many are linked to protein kinases (intracellular kinase domain in addition to extracellular ligand binding domain), or interact with an intracellular kinase (when activated).
What are the active/inactive forms of G protien?
Active: bound to GTP
Inactive: bound to GDP
*Why a switch? G protein does not stay active for long. Turns itself off.
Details:
G protein is activated by dumping GDP and picking up GTP in response to some signal. NOT activated by phosphorylation of hte bound GDP to GTP.
G protein inactivates itself by catalyzing hydrolysis of GTP to GDP.
G protein should be able to catalyze:
a) phyosphorylation of GDP –> GTP
b) hydrolysis of GTP –> GDP
c) both
d) either one
b) hydrolysis of GTP –> GDP
When triggered by the appropriate receptor, a G protein binds GTP, which displaces a GDP. The GTP is then quickly hydrolyzed to GDP by the G protein itself, which is a GTPase.
Why are ser and thr used by protein kinase to add phosphate.
Ser and Thr are the only AAs with free hydroxyls in their side chains. OH is needed to form an ester bond with a phosphate.

Which type of hormone would you find circulating freely in blood? Which would be bound to a protien?
Peptide hormones
or
Steroids
Peptide hormones have hydrophilic regions–> can circulate free in blood.
Steriods are lipids and hydrophobic (lipophilic). To prevent them from forming fat globules in the watery blood, they are carried in association with the hydrophilic proteins.
Which is faster acting, epinepherine or a cortisol?
Epinepherine induces a more rapid response, bc simply activates certain proteins that already exist in the cell. Making new proteins from scratch takes longer, wich is the case with steriod hormones like cortisol.
What do steroids look like?
Lipid soluble:
greasy/oily phase /dirty


