Separate Chemistry - C1 Atomic Structure and the Periodic Table Flashcards
What is the name of the smallest part of an element that makes up all substances?
An atom
What do all chemical symbols,
e.g. Na and O, have to start with?
A capital letter
Approximately how many different elements are there?
There are around 100 different elements.
What is a compound?
- Two or more elements
- Chemically combined
How can a compound be separated?
By a chemical reaction
What is a mixture?
Two or more elements, or compounds, that are not chemically bonded together.
What separation process is shown in the diagram?

Crystallisation
What type of separation process is shown in the diagram?

Filtration
What type of separation process is shown in the diagram?

Distillation
What type of separation process is shown in the diagram?

Fractional Distillation
What type of separation process is shown in the diagram?

Chromatography
Describe how you could use chromatography to identify a banned food dye in food colouring.
- Place food dyes on pencil line on chromatography paper.
- Place paper in solvent (e.g. water)
- The dye will dissolve in the water and spread up the paper.
- The colours in the dye will separate.
- Compare the patterns of the food dyes with the chromatogram of the banned food dye.
Why might a scientist decide to change or replace an existing model?
New experimental evidence is put forward
The discovery of what sub-atomic particle led to the plum pudding model of the atom?
The electron
Can you correctly label the diagram of the plum pudding model below?

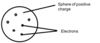
Name the experiment shown in the picture

Alpha Particle Scattering Experiment
What two conclusions about the atom were drawn from the results of the alpha particle scattering experiment?
- The mass was concentrated at the centre (the nucleus)
- The nucleus was charged.
Who adapted the nuclear model of the atom by suggesting that electrons orbit at specific distances?
Niels Bohr
How many protons, electrons and neutrons are in the following elements?
*these are just examples, you need to be able to do this for any element by using your periodic table

Sodium (Na): 11 protons, 11 electrons, 12 neutrons
Nitrogen (N): 7 protons, 7 electrons, 7 neutrons
Fluorine (F): 9 protons, 9 electrons, 10 neutrons
Calculate the relative atomic mass of chlorine given the percentage abundances of the following isotopes of Chlorine:
75 % Chlorine-35 and 25% Chlorine-37
*this is just an example, you need to be able to do this for any element – you will be given the percentage abundances of isotopes
(75% x 35) + (25% x 37)
= 35.5
Write and Draw the electron structures of the following elements:
*these are just examples, you need to be able to do this for any of the first 20 elements by using your periodic table
Na: (2,8,1)
F: (2,7)

Why is the table of elements called a ‘Periodic Table’?
Because elements with similar properties occur at regular intervals.
Q. What are the columns in the Periodic Table called?
Q. What are the rows in the Periodic Table Called?
Q. How are the elements in the Periodic Table arranged?
- Groups
- Periods
- Atomic (proton) number
What similarities are there between elements in the same group?
- Same number of electrons in outer energy level/shell
- Similar Properties






