rheumatoid arthritis Flashcards
what is rheumatoid arthritis *
NOT a condition of old people
it is a chronic automimmune disease
characterised by pain, stiffness and symettrical synovitis (inflammation of the synovial membrane) of synovial (diarthrodial) joints (the free moving joints)
usually aged 30-50yrs
clinical features of rheumatoid arthritis *
chronic arthritis
- POLYarthritis - swelling of the small joints of hand and wrist is common (poly means >5 joints affected)
- symettrical - eg both L and R hand evenly
- early morning stiffness in and around joints - for long periods (hrs)
- may lead to irreversible joint damage and destruction if untreated = loss of function = impact life - these are joint erosions on radiographs
extra-articular disease can occur
- rheumatoid nodules under skin
- vasculitis
- episcleritis (eye)
- affect lung
rheumatoid factor can be detected in blood
- IgM autoAb against IgG Ab - this IgM autoAb is diagnostic, but can be found in other conditions so depends on the context
epidemiology of rheumatoid arthritis
1% population affected - relatively common cause of significant disability in young adults
more common in females - effect of female hormones on immune system
describe the genetic component in rheumatoid arthritis *
higher concordance for identical to non-identical twins
heritability estimates up to 60%
specific HLA-DRB gene varients mapping to aa 70-74 of the DRbeta-chains are associated with rheumatoid arthritis
there are a number of HLA-DRB alleles associated - they all encode a shared aa sequence in the HLA-DR antigen binding groove - this is called a shared epitope
describe the environmental component of rheumatoid arthritis *
smoking - contributes to 25% of population attributable risk
it interacts with the shared epitope to increase the risk
what are the joints most commonly affected by rheumatoid arthritis *
MCP
PIP
wrost
knee
ankle
MTP
(shoulder can be affected)
early disease in hand and feety
describe the features of RA *

swelling over the MCP joints and PIPJS
deformity in toes
callus formation under head of metatarsals due to joint deformity
these are late stage pictures
describe the joint damage and destruction seen in RA *
swan neck deformity - hyperextension at PIP and hyper-flexion at DIP
boutonniere deformity - button like - hyper-flexion at PIPJ
xray - subluxation look like dislocation of the MCPJ, bilateral ulnar deviation of the fingers

describe the pathology in the synovium (primary site of pathology for RA) *
in synovial joints - PIPJ synovitis (swelling) - palpate the swelling = soft
in tensynovium surrounding the tendons - extensor tenosynovitis - swelling on back of hand - cant fully extend lttle and ring fingers because of extensor tendon damage
in bursa - olecranon bursitis

describe subcutaneous nodules *
there is a central area of fibrinoid necrosis surrounded by histiocytes (macraphage lineage) and peripheral layer of connective tissue
occur ion 30% of pts
associated with sever disease, extra-articular manifestations and rheumatoid factor
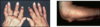
rheumatoid nodule - ulnar border of forarm is typical position - if present confirms RA and is invariably associated with rheumatoid factor
in hands - common location - around PIP joints
describe rheumatoid factor *
Ab that recognise the Rc part iof IgG
they are typicall IgM anti-IgG Ab
they form an immune complex - this activates complement - trigger inflammation
positive in 70% of disease at onest and further 10-15% become positive over the 1st 2 yrs of diagnosis
if positive called sero+ve RA
sero -ve is better prognosis - inflammation is more mild
describe the Ab to citrullinated protein Ag in RA *
Ab to citrullinated peptides (ACPAs) are highly specific for RA - anti-cyclic citrullinated peptide Ab (anti-CCP Ab)
citrulline is not found in normal proteins - created by convergion from arginine with enzyme: peptidyl arginine deiminases (PADs)
PADs are present in high concentrations in neutrophils and monocytes and consequently there is an increased citrullination of autologous peptides in the inflammed synovium
ACPA is strongly associated with smoking and the HLA shared epitope
teh shared epitope preferentially binds non-polar aa like citrulline bot not +ve charged like arginine - so ACPA is more likely to develop among individuals with citrulinated autoag who have a shared epitope
smokers have chronic airwauy inflammation - increase citrulination of peptides - this triggers the autoimmune reponse
describe HLA molecules and RA *
individuals are suseptible to RA because they carry the conserved aa sequence in their HLA-DRbeta ag-binding groove (shared epitope)
this epitope preferentially binds to citruline and citruline containing peptide antigens increased during inflammation
HLA-DRbeta is a class 2 allele - so presents to CD4 T cells - suggesting T cell involvement in the pathogenesis
the epitope is responsible for presenting specific peptides to T cells
what are the extra-articular features of RA *
malaise, fever, weight loss, SC nodules
uncommon:
vasculitis - inflammation of bv - can lead to tissue ischemia and necrosis of finger tips
ocular inflammation - episcleritis - red area of eye
neuropathies - inflammation damage peripheral nerves = weak/loss of sensation of hand/foot
amyloidosis - inflammation for a long time get increased serum amyloid a - can deposit in organs and cause organ failure eg renal/enlarged spleen
lung disease - nodules (do chest xray), fibrosis (SOB - fibrosis also caused by methotrexate so need to determine if drug or RA causing it), pleuritis (inflammation of plura - pain on inspiration)
fetty’s syndrome - triad of splenomegaly, leukopenia and RA
describe the radiographic abnormalities for RA *
very early - normal - want to catch it here - once see signs start to have missed boat for treatment
early - juxta-articular osteopenia - thin bone - reduced white colour
later- joint erosions at margins of the joint
later still - joint deformity and destruction
all carpal bones coelece, in MCPjs - less joint space and irregular, erosion, erosion of ulnar styloid, ragid irregularities of MCPjs


