Renal handling of acid-base (acid-base mechanisms) week 2 Flashcards
General guidelines: T or F: Acid/base input must equal acid/base output.
True. Acids and bases are subject to the same “input = output” balance considerations as other substances we have described (H20, Na+ , K+ , urea, etc.). Normally, the body gains ~60mEq/day of acid and thus to maintain balance the kidneys excrete this amount of acid per day. As with other substances, a disturbance may upset the existing balance but mechanisms will kick in and work toward regaining balance.
General guidelines: How is pH calculated? What is the normal plasma pH?
Acidity is measured in terms of pH. In words, pH is the negative log of the free hydrogen ion concentration ( pH = -1 x log[H+] ). For example, a pH of 4 means that there are 10-4 moles/L of free H+ present (recall log(10-4)= -4 ). A pH of 7 means that there are 10-7 moles/L of free H+ present. The pH of plasma is tightly regulated around 7.4 normally.
General guidlines: Body fluids are buffered. What is a buffer and how do they work?
Body fluids are buffered. A buffer prevents large changes in pH when free H+ is added to (or removed from) the system. Buffers do this by binding (or unbinding) protons (H+ ). For example, you add new free H+ to a beaker containing a buffer. The buffer will bind most of the added H+ leaving only a very small amount of the added H+ still free. The result is that the pH (free H+ level) does not change much (the added acid was buffered). The cost is that some of the buffer was consumed (i.e. is no longer available to bind free H+ ). In other words, the buffering capacity of the solution is reduced.
General guidelines: What is the most important pH buffer in the body?
What is the rxn for this buffer?
What enzyme is involved?
Why is the buffer capacity of this system so large?
The most important pH buffer in the body is the C02-bicarbonate system. The kidney accomplishes most of its role in acid-base balance through this system. The C02- bicarbonate system is summarized below.
C02+ H2O <–> H2CO3 <–> H+ + HCO3-
This reaction is freely reversible. C02 and H20 are abundant so the buffer capacity of the system is very high. The combination of C02 and H20 into carbonic acid (H2C03) is catalyzed by an enzyme called carbonic anhydrase. The reactions are quite fast and the amount of carbonic acid present is usually minuscule (to the point that it can usually be ignored).
General guidelines: Why is CO2 considered a volatile acid?
What is a fixed acid? Give examples.
C02 is not an acid per se (it can not by itself donate a free H+ ). But, it reacts with H20 (see above) and this reaction can generate a free H+ . Since H20 is ubiquitous in the body, C02 acts “in effect” as an acid. Indeed, C02 is often referred to as a volatile acid (because it can escapes from solution as a gas). In contrast, many other acids in the body are fixed (e.g. lactic acid, sulfuric acid, etc.). Many of these fixed acids are produced metabolically.
General guidelines: How do gains and losses of fixed acids affect bicarbonate levels? Be specific.
Gain and loss of fixed acids to/from the body alter bicarbonate levels. Adding free H+ (acid gain) drives the reaction above to the left reducing the bicarbonate (HCO3 - ) level on an almost molecule-for-molecule basis. Removing free H+ (acid loss) drives the reaction above to the right elevating HCO3 - level. Although there are many ways the body can gain and loss fixed acid, the result will always be the same (i.e. a change in HCO3 - level). The following is provided to help clarify this point further.
Acid Gain: Imagine a process that puts free H+ into the blood. Most of it will bind to the buffer present (HCO3 - ) and there will be little pH change. This binding creates carbonic acid that quickly dissociates to C02 and H20. Thus, the net result of the adding the acid is loss of HCO3 - .
Acid Loss: Image a process that takes free H+ from the blood. C02 and H20 will combine to create carbonic acid that quickly dissociates to HCO3 - and H+ . This free H+ replaces the one originally taken but this also results in a gain of HCO3 - .
Bottom Line: Gain/loss of fixed acid alters the body’s HCO3 - levels. This essentially transforms the problem of maintaining routine acid balance into one of maintaining HCO3 - balance. This is where the kidney plays a role.
General guidelines: Plasma pH is defined by the ration of what 2 molecules? What equation is used to determine plasma pH?
The plasma pH is defined by the ratio of C02 and HCO3 - levels. This is best expressed in the Henderson-Hasselbalch equation.
pH= pKa + log [A-]/[HA]
pH= 6.1 + log [HCO3-]/[0.03 x PCO2]
The 0.03 is the solubility factor which converts C02 partial pressure into mM.
What are the normal values of HCO3- and PCO2?
What organs are responsible for controlling HCO3- and PCO2?
The normal arterial value of plasma HCO3 - is 24 mM and normal arterial PCO2 is 40 mm Hg. Since 24/(0.03x40) is 20, normal plasma pH is 6.1+Log20 or 7.4. Remember, plasma pH is defined by the ratio of C02 and HCO3 - levels. The lungs control PCO2. The kidneys control [HCO3 -].
What are the 3 sources of the body’s daily acid load?
How much acid per day do the kidneys have to handle to achieve acid/base balance?
Describe the filtered load of H+. How does most of acid handled by the kidney get into urine?
Generally, how is most acid excreted in urine? (what form)
The origins of the body’s daily acid load are the food consumed, the body’s metabolism as well as the base lost in feces. Remember that the loss of base is equivalent to the production of acid. Each of these contributes ~20 mEq/day. This means the kidneys have to deal with a total of ~60 mEq/day acid per day to achieve balance. This is graphically illustrated in Figure 7.1 (attached).
At the nephron level, the filtered H+ load per day is very small. It can be calculated from plasma pH and normal daily GFR (7.4 & 180 L/day, respectively). A pH = 7.4 represents a free H+ concentration of 40 nEq/L (for those interested 40 nEq/L = 10-7.4 Eq/L). Thus, the filtered H+ load per day is 7200 nEq/day (calculated as 40 nEq/L x 180 L/day). Changing nEq to mEq, the filtered H+ load is 0.0072 mEq/day!! This is more than 8000 times less than the ~60 mEq acid that enters the body per day. The point is that H+ filtration alone can not possibly account for the amount of acid excreted in the urine per day. The only way ~60 mEq/day acid can get into the nephron (in order to be excreted) is by secretion. Another important point is that most of the ~60 mEq/day acid that appears in urine is not in the free H+ form. A person would have to produce 2 L/day of urine with a pH of ~1.5 if all the acid were in the free H+ form. Urine pH is rarely less than 4.5 and so most (>99%) excreted H+ is bound with other substances.
Describe the filtered load of HCO3- per day.
Why is reabsorption of HCO3- so important?
Where in nephrons does most of HCO3- reabsorption take place? Where else is HCO3- reabsorbed?
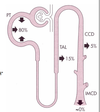
Bicarbonate is freely filtered. The typical plasma HCO3 - level is 24 mM (or 24 mmoles/L). Since daily GFR is 180 L/day, 4320 mmoles/day enters Bowman’s space by filtration. Loss of this quantity of base (if it all were excreted) is equivalent to the body gaining 72 times its normal daily acid intake. Thus, renal acid/base handling must start by assuring that most (if not all) the filtered HCO3 - is reabsorbed. Figure 7.2 illustrates the percentage of the filtered HCO3 - load that is typically reabsorbed by different tubular segments. Most filtered HCO3 - (80%) is reabsorbed from the proximal tubule.
Explain, in detail, the mechansim of HCO3- reabsorption in the proximal tubule.
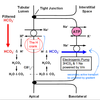
In the proximal tubule, most bicarbonate reabsorption occurs via the transcellular route. This proximal HCO3 - reabsorption involves secretion of H+ by an apical Na-H-antiporter. The Na+ gradient driving the Na-H-antiporter is established by the basolateral Na-K-ATPase. Besides providing the tubular H+ required for HCO3 - reabsorption, the Na-H-antiporter is also an important factor in proximal Na+ reabsorption. The secreted H+ associates with a filtered HCO3 - and forms carbonic acid. This carbonic acid then dissociated into CO2 and H2O. These can easilypermeate across the apical membrane and may re-associate inside the cell to again form carbonic acid. Carbonic anhydrase catalyzes these reactions. Inside the proximal tubule lumen, the carbonic anhydrase is tethered to the lumenal surface of the apical membrane. The carbonic acid formed in the cell then dissociates into H+ and HCO3 - . The H+ may find its way back to the NaH-antiporter and be secreted again. The intracellular HCO3 - is moved across the basolateral membrane by the Na-HCO3 symporter.
It is interesting to note that the HCO3 - that ends up in the interstitial space was never actually in the tubular lumen. Nevertheless, a HCO3 - in the tubular lumen disappeared and another HCO3 - , which formed in the cell, ended up in the blood. The net result is reabsorption of a HCO3 - molecule.
It is also interesting that the H+ secreted during HCO3 - reabsorption is “in effect” recycled. It does not end up being excreted and thus does not contribute to acid excretion. Instead, it is combined into H2O and intracellular formation of HCO3 - spawns another H+ inside the cell
What cells in the cortical collecting ducts reabsorbed HCO3-?
Explain, in detail, the mechanism of HCO3- reabsorption in these cells.
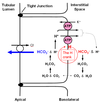
Remember there are different kinds of cells in the cortical collecting duct. Bicarbonate is reabsorbed by type-A intercalated cells. Figure 7.4 (attached) shows HCO3 - reabsorption by a type-A intercalated cell.
The general blueprint of how HCO3 - is reabsorbed is similar in the proximal tubule and cortical collecting duct. Step 1 is apical H+ secretion. Step 2 is H+ association with a filtered HCO3 - . Step 3 is equilibration of CO2 across the apical membrane. Step 4 is formation of HCO3 - inside the cell. Step 5 is transport of HCO3 - across the basolateral membrane. There are two notable differences between HCO3 - reabsorption in proximal tubule and cortical collecting duct. The first difference is the identity of the transport proteins involved. In the cortical collecting duct, apical H+ secretion is mediated by the H-ATPase or K-H-ATPase. The second difference is that basolateral HCO3 - transport in the collecting duct is mediated by the Cl- HCO3 antiporter. There is debate whether or not carbonic anydrase is present inside the tubular lumen of the collecting duct. If not (the traditional dogma), then the bicarbonate reaction can still proceed but at a slower rate. If it is present (not yet definitively established), then it appears to be an isoform different than that found in proximal tubule. The important things for you to remember here are that, 1) the overall scheme of how HCO3 - is reabsorbed is similar in proximal tubule and cortical collecting duct but, 2) the identity of the transport proteins involved are different.
Overall, the nephron is capable of reabsorping almost all HCO3- that is filtered.

If there is excess base, what 2 general processes occur to rid of excess base?
When there is excess HCO3 - present, the extra HCO3 - will be excreted in the urine. The nephron can accomplish this 2 ways. First, it simply can reabsorb less of the filtered HCO3 - leaving some to be excreted in the urine. Second, it can secrete HCO3 - into the tubular fluid to be excreted in the urine.
Where does HCO3- secretion occur? What cells secrete HCO3-?
Explain the cellular mechanism of HCO3- secretion.
The site where HCO3 - secretion occurs is the cortical collecting duct. Above you were told the cortical collecting duct is the site of HCO3 - reabsorption. How can the same nephron region do both (secrete & reabsorb HCO3 - )? The answer is that different types of cells do the different things. Type-B intercalated cells secrete HCO3 - while type-A intercalated cells reabsorb HCO3 - . Of course, the acid/base status of the body determines which actually occurs. If there is too much HCO3 - in the plasma, then there will be net HCO3 - secretion into the cortical collecting duct. Figure 7.5 (attached) illustrates the process of HCO3 - secretion in the cortical collecting duct (by a type-B intercalated cells). Interestingly, the process of HCO3 - secretion by a type-B intercalated cell is essentially the inverse of HCO3 - reabsorption by a type-A intercalated cell. It is as if the type-B cells are just a “flipped-around” type-A cells.
As shown in Figure 7.5, a HCO3 - in the interstitium disappears and another HCO3 - (formed in the cell) is transported into the tubular lumen. Moving through the process stepwise…Step 1 is basolateral H+ pumping. Step 2 is H+ association with interstitial HCO3 - . Step 3 is equilibration of CO2 across basolateral membrane. Step 4 is formation of HCO3 - inside the cell. Step 5 is transport of HCO3 - across the apical membrane. The net result is HCO3 - secretion.
Where in the nephron does most H+ secretion occur? What cell type?
Explain the cellular mechanism of H+ secretion in this portion of the nephron.
In what 2 ways does H+ secretion by the kidneys alkalinize plasma?
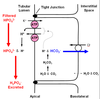
H+ secretion occurs in Type A intercalated cells of the cortical collecting duct. These are the same cells that reabsorb bicarb.
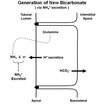
Incredibly, the kidney makes new HCO3 - to replace the HCO3 - lost from the plasma. Briefly, H+ is actively secreted and it complexes with a buffer other than bicarbonate in the tubular lumen. The H-complexed base is eventually excreted in the urine (i.e. the H+ is lost). Apical H+ secretion (as it does during HCO3 - reabsorption) promotes formation of HCO3 - inside the cell and this HCO3 - is then transported into the interstitium. The difference here is that this HCO3 - is new, not just a replacement for HCO3 - “consumed” in the tubular lumen. Thus, the net result is H+ excretion and the appearance of new HCO3 - in the plasma. What is this “other than bicarbonate” buffer that complexes with the secreted H+ . The main “other than bicarbonate” buffer in the tubular lumen is phosphate. Figure 7.6 (attached) graphically shows the role of phosphate (specifically HPO4 2-) in H+ excretion and generation of new HCO3 - .
It is interesting to compare Figure 7.6 (Generation of New Bicarbonate) and Figure 7.4 (Bicarbonate Reabsorption). The difference between the two figures is what the secreted H+ complexes with in the tubular lumen. In Figure 7.6, it complexes with phosphate and the result is generation of new HCO3 - . In Figure 7.4, it complexes with a filtered HCO3 - and the result is HCO3 - reabsorption. Another way to explain this is to start by stating the fact that a HCO3 - is always generated inside the tubular cell when a H+ is secreted. If the secreted H+ complexes with filtered HCO3 - , then the HCO3 - that forms inside the cell is simply a replacement for the one that disappeared in the filtrate (i.e. in the tubule lumen). As a result, there is no change in the number of HCO3 - in the system. If the secreted H+ complexes with filtered HPO4 2-, then the HCO3 - that forms inside the cell it is not replacing a HCO3 - that disappeared in the tubular lumen. In this case, the number of HCO3 - in the system has increased (i.e. there is new HCO3 - ). Another important point here is that renal generation of each new HCO3 - is associated with the eventual excretion of one H+ ion. Note that this excreted H+ ion is not free but instead it is bound to a buffer (i.e. phosphate). New HCO3 - generation with simultaneous H+ excretion will alkalinize the plasma (make it less acidic).
What is a titration?
Why is phosphate considered at titratable acid?
Explain the importance of phosphate as a titratable acid. What other titratable acids are exist?
In the lab, the procedure of carefully adding measured amounts of acid or base to a buffer solution is called a titration. In the renal tubule, H+ secretion essentially “titrates” HPO4 2- to H2PO4 - (which is excreted) and drives production of new HCO3 - (as described above). The phosphate is a titratable acid. This is best explained as follows. Imagine we are testing a urine sample and we want to estimate how much new HCO3 - was produced due to secreted H+ ions binding with phosphate. To do this, we will titrate the urine sample with a base (like NaOH) until the urine’s pH is equal to that of plasma (7.4). The added base will transform H2PO4 - back into HPO4 2- (reversing what happened in the renal tubule). The amount of base we have to add will be equal to the amount of H+ that was secreted into the renal tubules. This latter value is the titratable acidity of the urine. Phosphate is by far the most abundant and important titratable acid. However, it is not the only titratable acid. Other substances (like creatinine) can act as titratable acids but their contribution is usually very minor.
What other portion of the nephron secretes excess H+?
What molecule is used to rid of excess acid in this area of the nephron? Why is another molecule (other than HPO4-) needed to secrete excess acid?
When is this molecule used to rid of excess acid?
Explain the cellular mechanism of H+ secretion in this portion of the nephron.
What is the end result of this H+ secretion?
Normally, the amount of acid excreted in urine that is associated with phosphate is ~40 mEq/day. The body typically gains ~60mEq/day of acid and thus another ~20mEq/day of acid typically needs to be excreted in order to maintain acid/base balance. As stated before, most excreted H+ (>99%) is bound with other substances in the urine. The two main substances that excreted H+ (>99%) is bound to are phosphate (see above) and ammonium. Acid secretion via ammonium is a complicated topic with many tricky elements. Explaining it fully here is not practical so several “short-cuts” are taken in order to present it in a concise manner.
When the body needs to rid itself of acid, NH3 is deliberately produced from the amino acid glutamine in proximal tubule cells. Each NH3 produced quickly diffuses across the apical membrane and binds with a secreted H+ ion. This produces NH4 + which is eventually excreted (carrying the bound H+ ion with it). The process is enormously more complex than this but this is “in effect” how it works. The end result is that one new HCO3 - enters the blood for each H+ ion excreted via NH4 + . Remember, new HCO3 - is produced whenever a secreted H+ binds to something other than filtered HCO3 - in the tubular lumen (here its NH3 before it was phosphate). The catalysis of glutamine here is “on demand”. Thus, the situation presented in Figure 7.7 occurs only when the body needs to rid itself of acid. It does not occur at other times.
Why is ammonium not considered a titratable acid?
Ammonium (NH4 + ) is formed by H+ binding to ammonia (NH3). Ammonium is an acid that dissociates fully only at very basic pH’s. It is not a “titratable acid” because titrating an acidcontaining urine sample to pH 7.4 with a base (as described above) will not dissociate the bound H+ . In other words, it is not a “titratable acid” because the H+ stays bound over the normal range of urine pH’s.
How is net HCO3- gain/loss calculated?
State what the normal values (in mEq/day) of urine, HCO3-, titratable acid, urine NH4+, and urine pH in the following circumstances:
normal
alkalosis
acidosis
Maintaining acid-base balance is essentially maintaining the body’s HCO3 - balance. The nephron reabsorbs filtered HCO3 - , sometimes secretes it and can even produce new HCO3 - (as discussed in the various sections above). To “see” the net affect of all these processes together, the following values are needed.
One: Amount of HCO3 - in the urine.
Two: Amount of “titratable acid” in the urine. This is equal to the amount of new HCO3 - generated via H2PO4 - excretion.
Three: Amount of ammonium in the urine. This is equal to the amount of new HCO3 - generated via NH4 + excretion.
Net HCO3- gained or loss by the body is then:
Net HCO3- Gain/Loss = Total New HCO3 - (via H2PO4 - and NH4 + ) - Urine HCO3
Some example urine data for these values are shown in the TABLE BELOW (prepared by Dr. Fill, attached). Note that no data for free H+ concentration is provided. This is because the number of free H+ ions in even very acidic urine (pH 4.5) is trivial to the overall picture here.
Note the acidosis numbers. Excretion of NH4 + plays a much bigger role than titratable acids when there is a substantial acidosis.

What signals H+ secretion to increase or decrease?
What signals H+ secretion to increase or decrease? The answer is plasma CO2 level and arterial blood pH. How plasma CO2 level and arterial blood pH do this is not well understood. It appears that these signals act either on or in the renal epithelial cells to up-regulate or down-regulate H+ transport in the various sections of the nephron. How the signals are sensed is not clear. How the signals are transduced into cellular action is also unclear.
The bottom line is that the epithelial cells in the renal tubules respond appropriately when plasma CO2 level and/or arterial blood pH change.
At what pH is acidosis clinically defined as?
What does respiratory acidosis result from?
What does metabolic acidosis result from?
What conditions may cause each type of acidosis?
Explain (generally) how the values of the Henderson-Hasselbach change in acidosis.
Acidosis is clinically defined as a condition when the plasma pH is less than 7.35. Acidosis can be caused by two general categories of disturbances:
- Respiratory acidosis results from high plasma PCO2. Reduced respiration leads to an increase in arterial PCO2 and hence a decrease in pH. Examples of the sorts of conditions that can produce respiratory acidosis include emphysema, muscular dystrophy, multiple sclerosis and sleep apnea.
- Metabolic acidosis results from low plasma HCO3 - concentration. Conditions that can result in metabolic acidosis include diabetes, lactic acidosis, diarrhea, and advanced renal failure (thus metabolic acidosis can be of renal origin).
It is very important to recognize that neither the absolute value of plasma HCO3 - concentration or of PCO2 alone determines plasma pH. Instead it is their ratio that determines pH. Remember the Henderson-Hasselbalch equation,
pH= 6.1 + log [HCO3-]/[0.03 x PCO2]
Normally (plasma pH = 7.4), the ratio [HCO3 -]/(0.03xPCO2) is equal to 20. In an acidosis, this ratio is less than 20 either because plasma [HCO3 -] is lower than normal or because PCO2 is higher than normal (or occasionally both). Of course, renal compensation can not directly affect PCO2 (this is the job of the respiratory system). Renal compensation involves changes in the secretion of H+ and reabsorption/production of HCO3 - . In the case of acidosis, renal compensation will involve more secretion of H+ and hence more excretion of NH4 + and titratable acids. Also, essentially all filtered HCO3 will be reabsorbed.
At what pH is alkalosis clinically defined as?
What does respiratory alkalosis result from?
What does metabolic alkalosis result from?
What conditions may cause each type of alkalosis?
Explain (generally) how the values of the Henderson-Hasselbach change in alkalosis.
What is the renal response to alkalosis?
Alkalosis is clinically defined as a situation in which plasma pH is greater than 7.45 (the normal range of plasma pH is 7.35 to 7.45). Alkalosis can also be either of respiratory origin (low PCO2) or metabolic origin (high HCO3 - ). Respiratory alkalosis results from lower than normal PCO2 and this can result from hyperventilation (for example). Metabolic alkalosis results from higher than normal plasma HCO3 - concentration. This can result from (for example) vomiting, hyperaldosteronism and Cushing’s’ disease. During alkalosis, the ratio [HCO3 -]/(0.03xPCO2) will be greater than 20. This means that plasma pH is above normal. The renal response to an alkalosis of either respiratory or metabolic (non-renal) origin is to generally reduce the reabsorption of HCO3 - (i.e. increase excretion of HCO3 - in urine) and reduce production/excretion of NH4 + and titratable acids (i.e. decrease new HCO3 - production by the kidney). The overall effect of this is to reduce plasma HCO3 - concentration and hence move the pH back towards normal.
What sub-category of acid-base disturbances are those of renal origin?
In addition to acidosis and alkalosis of metabolic or respiratory origin as very briefly described above, there can also be acid-base imbalances of renal origin. These are, in fact, a sub-category of metabolic acidosis or alkalosis conditions. These are caused by a disorder in the kidney itself. For example, the kidneys capacity to resorb or secrete HCO3 - may be impaired for some reason. It is important to distinguishing among the possible origins of acid-base disorders. These origins are:
- Respiratory (acidosis/alkalosis)…. due to abnormalities of ventilation.
- Non-renal Metabolic (acidosis/alkalosis)…. due to any cause other than the lungs or the kidney.
- Renal Metabolic (acidosis/alkalosis)…. due to a disorder in the kidney itself.
SEE SLIDES 14-15 OF NOTES
What is anion gap? How is it calculated?
What is the normal range for anion gap?
How does the addtion of a fixed acid affect the anion gap?
The so-called “anion gap” is a concept that is important in clinical nephrology when acid-base disorders are considered (and will very likely be revisited in your future Pathophysiology courses). Before going further, it is important to understand that there is never a measurable difference (gap) between total anions and cations in the plasma or elsewhere in the body. The concentration of all negative (anions) and positive (cations) charges in any compartment in the body is the same (very tiny differences exist but these can not be measured and they have nothing to do with what is called the “anion gap”). The anion gap is defined (in its simplest form) as the difference between the concentration of the major plasma cation (Na+ ) and the major plasma anions (Cl- and HCO3 - ). This can be expressed as:
Anion gap = [Na+]PLASMA – ([Cl-]PLASMA + [HCO3 -]PLASMA)
Under normal conditions the anion gap is typically ~12 mM with the normal range considered to be 8 to 16 mM. The normal values for the plasma concentration of Na+ , Cl- and HCO3 - are:
Na+ : 140 mM Normal range being 136-145 mM
Cl- : 102 mM Normal range being 98-106 mM
HCO3 - : 24 mM Normal range being 22-26 mM
These numbers determine the typical value and normal range for the anion gap. However, when a fixed acid (not a volatile acid) is added to the body the concentration of free H+ ion will increase (only slightly due to buffers) and the concentration of HCO3 - will decrease (much more significantly). In the plasma, the fixed acid dissociated to produce a free H+ ion and an anion (e.g. lactic acid <–> lactate + H+ , lactate is an anion). This newly produced anion in the plasma displaces some Clor HCO3 - in the plasma and thus changes the anion gap.


