Proteins Flashcards
structure of an amino acid?

Which form are all amino acids in ?
L optical isomer
- the alpha carbon is the chiral centre and four different things bond to the central carbon
- this gives rise to optical isomers
glycine is the only nonchiral amino acid as it has no side chain
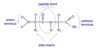
what are the characteristics of a peptide bond?
- there is no free rotation
- C – O and N-H are in the same plane
- Other two bonds in the backbone of the peptide are able to rotate
- Only conformation in which the side chains do not clash with the main chain (steric hindrance) are allowed
how are peptide formed?
what is the anatomy of a peptide?
- formed by condensation reactions
- functionality requires a definite 3D structure or conformation of the polypeptide chain
Hydrophobic
Glycine Alanine Proline Valine Leucine Isoleucine Tryptophan Phenylalanine, Methionine
giant ants take lollipops inside picnics, maybe vinegar pringles?
giant anal prolapses vary
leucine and isoleucine may take pharmacology
Hydrophilic
Serine Thereonine Tyrosine Asparagine Glutamine Cysteine
small timid tigers can grab ants
sometimes terrorizing teachers acquire giant cysts
amino acids with positively charged side chain
Arginine Lysine always protonated at physiological pH
Histidine is protonated (+) under pH 6
Negatively charged
Glutamate Aspartate are always negatively charged at physiological pH
Why can proteins not tolerate a great change in pH?
Ionisation state of amino acids is essential for function. Changes in pH = change in ionisation state = change in property.
Amino acids can take up and release protons which gives them some buffering capacity = gives them capacity to resist some changes in pH
what hold a protein together?
what bonds?
covalent bonds
hydrogen bonds
ionic interactions
van der waals forces
hydrophobic interactions
When do disulphide bridges form?
Cytosine chains are oxidised
Covalent bond between 2 amino acids
explain covalent bonds
- The strongest bonds within a protein and exist in the primary structure
- Covalent bonds can also exist as DISULPHIDE BRIDGES
explain hydrogen bonds?
- Occur when two atoms bearing partial negative charges share a partially positively charged hydrogen
- These can occur between atoms on different side chains and the backbone of the protein OR between water molecules
explain ionic interactions?
- Arise from the electrostatic attraction between CHARGED SIDE CHAINS
- Relative STRONG bonds
- Within any particular protein molecule, the majority of charged groups are at the surface of the folded protein - there they can be neutralised by counter ions such as salts
explain van der waals forces?
- Transient, weak electrostatic attractions between two atoms - due to the fluctuating electron cloud surrounding each atom which has a temporary electric dipole
- the transient dipole in one atom can induce a dipole in the other atom
- The appropriate distance required for Van der Waals attractions differs based on the size of each electron cloud - referred to as the Van der Waals radius
- because there are lots of van der waals forces they can be strong all together
explain hydrophobic interactions?
- They put hydrophobic side chains close together by packing them into the interior of the protein
- This creates a hydrophobic core and a hydrophilic surface to the majority of proteins
Why does proline cause a kink in the peptide chain?
- When proline is added to a polypeptide chain there is a loss of NH group of the amino acid
- Side chain can’t form hydrogen bonds with C=O of another part of chain
- this distorts the helical conformation
- it puts a kink into it
how are proteins usually folded?
- proteins are generally folded into the single conformation of the lowest energy
- Chaperones may be involved to make sure that folding occurs in the most energetically favourable way
what does functionality require?
requires a clear 3D structure or conformation of the polypeptide chain
What is a parallel beta strand?
- Beta sheets run parallel to each other
Beta sheets run in opposite directions = antiparallel beta sheet
the pleating in each case allows the best alignment of the hydrogen-bonded groups
how are beta pleated sheets held together?
how are alpha helices held together?
- Hydrogen bonds between the C=O and the N-H of two or more beta strands hold the entire structure together
- they are held together by hydrogen bonds
what is a domain?
compact globular structure that the secondary structure motifs are arranged into
what is denaturing
what are common denaturants?
- urea breaks hydrogen bonds
- 2-mercaptoethanol (breaks disulphide bonds)
what is the post-modification of proteins?
- the starting set of 20 amino acids can be modified to create novel amino
acids enhancing the capabilities fo the protein
- The formation of g-carboxyglutamate residues within several proteins of the blood clotting cascade is critical for their normal function by increasing their calcium binding capacities


