Principles of Diffraction Flashcards
(66 cards)
Describe diffraction.
Diffraction is the process by which a system of waves is spread out by interaction with matter.
If we can create a lens, e.g. in a microscope, then the diffracted waves can be used to recreate an image. Otherwise, we can only measure the waves and reconstruct using a computer e.g. with x-rays and other forms of radiation.
What is the 2-slit (2-atom) experiment?
- when there’s one origin of diffraction, we get one source of waves spreading out from the origin in all directions
- when there’s light shining through a second slit/onto a second atom, we get another source of waves spreading out from the second origin
- when we have radiation spreading out from both sources at once we get interference between the waves
- in some directions the waves add (constructive) to give strong peaks and troughs
- in some directions the wave cancel (destructive)
- different sources give different patterns
Why use crystals?
Problem:
- any interaction with an atom, which can tell us about where that atom is, will also damage the atom
Solution:
- observe lots of copies of the molecule in the same orientation at once = a crystal
Describe waves.
Waves are defined by three properties:
- wavelength
- amplitude
- phase
Two of these correspond to properties of radiation when considered as particles:
- wavelength - related to photon energy (high E = short wavelength)
- amplitude - proportional to number of photons
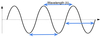
Describe wavelength.
The distance over which a wave’s shape repeats - also the distance between successive peaks or troughs.
For diffraction experiments, we work with monochromatic radiation so we can treat this as constant.
Describe amplitude.
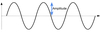
The amplitude of a wave describes how high the peaks and troughs are. It relates to the strength of the electromagnetic radiation, i.e. the number of photons. It is always positive.
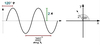
Describe phase.
The phase of a wave describes where the peaks and troughs are located (relative to some arbitrary reference position).
Phase is cyclic - if the wave is shifted by one complete wavelength then you get back to where you started. Therefore, phase is expressed as an angle - 360o gets you back to where you started.

How can waves be described mathematically?
For a given wavelength, a wave can be represented by two numbers: a positive amplitude and a cyclic phase.
The wave can therefore be written in terms of a trigonometric function using amplitude (A) and phase (φ).

Other than a trigonometric function, how else can a wave be described?
Amplitude and phase (angle) can also describe a vector in a plane using polar coordinates. Therefore, we can describe a wave by polar coordinates (A,φ) or in the complex plane by real and imaginary parts (Argand diagram).
Describe why understanding diffraction is important and what it entails.
To see atoms, we need to use short-wavelength radiation (or particles) but we don’t have a lens. Therefore, we need to understand the physics of diffraction to infer what arrangement of atoms would lead to a specific diffraction pattern.
Diffraction from two point scatterers:
- the incident beam is assumed to be coherent i.e. the waves are all in phase
- in the scattered beam, the wave may or may not be in phase
- if all are in phase = strong scattering in that direction
- if all are out of phase = no scattering in that direction
Describe the interference of waves aka what happens when multiple waves combine?
- if the phases match, we get reinforcement (constructive interference)
- if the phases differ by 180o then we get cancellation (destructive interference)
- if the phases differ by some other amount then we get a wave that is weaker than the amplitude sum and is of intermediate phase - the combined wave can always be determined by adding the vectors

Describe path difference and its effect on interference.
- Path difference = difference in distance travelled by the two waves from their respective sources to a given point on the pattern.
- When the path difference (d) is a whole number of wavelengths (Δ= nλ) we get constructive interference
- When the path difference is a half number of wavelengths (Δ = (n+1/2)λ) we get destructive interference

What is Bragg’s law?
Consider diffraction from crystal planes, rather than emission from 2 slits.
The diffracted (scattered) beams are in phase (i.e. strong diffraction) when the path difference is a whole number of wavelengths, i.e.
nλ = 2d sin(θ)
This is Bragg’s law.

What are the key points about Bragg’s law?
- Despite how they’re usually drawn, the atoms don’t need to line up; the path difference is the same wherever the atoms are on the planes. The only thing that matters is the distance between planes of similar atoms
- Scattering from subsequent layers of planes leads to increasing path differences represented by n > 1 in Bragg’s equation.
- if scattering from the first plane is in phase (n = whole number) then scattering from every subsequent plane will also be in phase
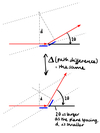
Describe a diffraction experiment.
- rotate the picture by θ to reflect the experiment
- the incident x-ray beam is fixed, but we can measure diffraction spots appearing in different directions around the crystal
- the angle of diffraction is 2θ
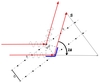
What is ‘s’?
- diffraction vector = difference between incident and reflected beams
- describes the position of the diffraction spot
- always perpendicular to the corresponding crystal plans
- the length of s is inversely proportional to plane spacing, d
Describe the angle of diffraction.
Angle of diffraction, 2θ, gets larger as the plane spacing, d, gets smaller.
I.e. the highest angle diffraction spots correspond to the closest together planes.
What are the components of an x-ray diffraction experiment?
- x-ray source
- filter/monochromator
- collimator
- sample/goniometer
- beamstop
- detector
What are the two ways of generating an x-ray source?
- bremsstrahlung/breaking radiation
- Siegbahn notation
Describe creating an x-ray source via bremmsstrahlung/breaking radiation.
- electromagnetic radiation is produced when a charge is accelerated
- x-rays can be produced by accelerating electrons from a cathode to an anode using a high voltage
- the deceleration of the electrons on hitting the anode gives off x-rays
- for higher intensities a rotating anode is used to limit heating of the anode by the electron beam
Describe creating an x-ray source via Siegbahn notation.
- characteristic radiation is produced when an outer shell electron fills a vacancy in an inner shell
- Cu and Mb are most used for lab sources
How can higher intensity x-rays be produced?
- synchrotrons provide much higher intensity (often tunable) x-ray sources for macromolecular crystallography
- originally from bending magnets which accelerate the electrons in a circle
- now, insertion devices such as undulators are used for even more intense radiation
Describe a filter/monochromator in an x-ray diffraction experiment.
- at a synchotron, a two-crystal monochromator uses Bragg diffraction to select different wavelengths by changing the angle of diffraction.
- e.g. a nickel filter will allow the Cu K-alpha radiation through while blocking the K-beta
How can you scan the diffraction pattern in an x-ray diffraction experiment?
- a four circle diffractometer has 4 angles which can be changed to excite different diffraction spots
- a mini-kappa goniometer is a simple version of the same concept
- originally, these were used with ‘point’ detectors, but area detectors allow us to produce 2D slices of the diffraction pattern
- a 3D crystal gives rise to a 3D diffraction patter, so with a 2D detector we technically only need to vary one more angle















