Inorganic Materials and Nanoparticles Flashcards
Describe crystallinity.
Solids exhibit a continuum of order from perfect crystals to totally amorphous.
- long range order = crystalline (e.g. SiO2 as quartz)
- short range order = amorphous (e.g. SiO2 as glass)
Describe structure types.
Give an example.
Structures of compounds can be divided into classes. Traditionally, each class is named after an archetypal compound or mineral.
e.g. ReO3 is built from vertex sharing ReO6 octahedra. Any other compound that exhibits this type of structure (e.g. NbF3) is called a structure type. NbF3 has ReO3 structure type.
Define polymorphism.
Some solid compounds can have more than one crystalline structure for a single stoichiometry, called polymorphism. Individual structures are called polymorphs.
Give an example of polymorphs and some key facts about polymorphs.
- Rutile, anatase and brookite are all polymorphs of TiO2
- individual polymorphs will be thermodynamically stable at a particular p and T
- other polymorphs can have kinetic stability at the same p and T
- often, a solid having a particular crystalline structure and stoichiometry is referred to as a phase
Give some general points on structure and charge of a bulk solid.
- for ionic solids, the anions are much bigger than the cations so the anions are close packed while the cations fit into the interstitial sites (the cations will be more mobile)
- in drawings, lines don’t always mean a bond they may describe coordination
- the overall charge of a bulk solid will be zero, however charge can be distributed heterogeneously
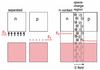
Describe general defects and non-stroichiometry.
- only at 0 K will an ionic crystal have a perfectly ordered array of atoms where every atomic lattice point contains an atom
- at > 0 K crystals contain defects
- defect formation requires energy and is always endothermic
- however, a structure with defects has higher entropy
- increased entropy drives defect formation to ΔG = 0
- strong T dependence on number of defects
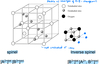
Describe intrinsic point defects.
- vacancy: an ion is missing
- interstitial: an extra ion is present in the interstitial site
- Schottky: a pair of vacancies are present in the lattice
- Frenkel: a defect pair is created by an ion (usually cation) moving into an interstitial site
- the lattice will distort at the vacancy site to minimise the loss in lattice energy
- vacancy and interstitial atoms do not have to be adjacent and can be mobile

What is the equation for the proportion of defects?

Describe the percentage of intrinsic point defects in ionic compounds.
- in most ionic compounds, the percentage of intrinsic point defects is small but they can have a significant effect on electrical, magnetic and optical properties
- the smallest ΔH (ΔHS or ΔHF) will determine if Schottky of Frenkel defects dominate
Describe extrinsic point defects.
Give an example.
- introducing different ions into the structure - known as doping
- Si (group 14) is not a good electrical conductor
- if Si is doped with small amounts of P (group 15) then the conductivity increases significantly
- an occupied state is introduced just below the Si conduction band
- after forming bonds to Si, the remaining electron from P acts as a conduction electron
Describe non-stoichiometry.
Give and example.
Defects and doping result in non-stoichiometric solids. It is common for compounds of transition metals that can have variable oxidation states.
e.g. TiO - rock salt structure over the range TiOX (0.7 < x < 1.25).
Non-stoichiometric compounds are distinct from other compositions e.g. TiO2 because they have a common structure. However, lattice parameters of the structure will change gradually as the ratio of elements changes.
Describe solid solutions.
Commonly observed in non-stoichiometric solids. A solid solution is a crystalline solid that can have continual varibale composition for a given structure type.
Describe the two types of solid solutions.
Substitutional:
- new atom replaces an existing atom e.g. by doping
- e.g. Al2O3 and Cr2O3 over the range (Al2-xCrx)O3 (0 < x < 2)
- similar size and charge allows Cr to occupy the same crystallographic sites as Al
Interstitial:
- an atom is added to an interstitial site
- e.g. C in Fe giving FeCx (0 < x < 0.09)
What is dynamics in solids?
- above 0 K, atoms and ions in compounds move and respond to external stimuli (e.g. magnetic/electric fields, pressure, temperature)
Lithium ion batteries are an example of long range movement of ions. What is this?
Batteries comprise of two electrodes and an electrolyte. The Li+ ions must be mobile in all the electodes and electrolyte materials.
Describe intercalation/deintercalation.
- species can be added/removed from a host structure
- needed for charge/discharge cycles
- important structural features: layers, channels or porosity provide space for Li+ to move
What are the equations for Ecell (V), power (W) and charge (Ah)?
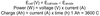
What are the equations for energy (Wh), energy density and specific energy?

What are the challenges in battery science?
- intercalation/decalation should not result in big structural changes - leads to mechanical stress, fracture and performance loss
- avoid uncontrolled Li metal growth (dendrites) = leads to shorting and probable ignition of flammable electrolye
- interfaces between electrodes and electrolyte are critical - growth of secondary layer of electrodes helps to prevent degradation of electrolyte (upon contact with strongly reducing and oxidising electrodes) and growth of Li dendrites
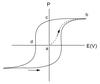
Descibe bulk polarisation.
When an ion is in an asymmetric site, there will be a local dipole moment.
To obtain bulk polarisation (P), the material must have a non-centrosymmetric crystal structure otherwise the local dipoles cancel out. Any process that changes the relative position of ions will change the polarisation.
What are the three different things that dipoles can respond to and what are they called?
- ferroelectrics - dipoles respond to an electric fireld
- piexoelectrics - dipoles respond to pressure
- pyroelectrics - dipoles respond to heat
Describe ferroelectrics.
- can retain polarisation (P) after the electric field has been removed
- have a very high dielectric permittivity, εr
Describe the properties of a good dielectric material.
- should have high dielectric strength (not breakdown at high voltages and become electron/ion conducting)
- have low dielectric loss (not lose electrical energy as heat in an alternating electric field)
- the stored charge can be measured in a parallel plate capacitor and the dielectric permittivity, εr, measured
Give an example of a ferroelectric.
BaTiO3 - has the perovskite ABO3 structure.
- at temperatures above 120oC, the Ti atoms are in a symmetric octahedral site (centrosymmetric)
- between 5 - 120oC, the Ti atoms are displaced along on of the axes of the octahedron and polarisation results (non-centrosymmetric)