Pigments and tissue deposits, developmental anomalies, neoplasia part II, bones Flashcards
(331 cards)
Matching! Which of these are pigments and which are tissue deposits?
A. Pigments
B. Tissue deposits
- Hematogenous pigments (hemoglobin, hemosiderin, bilirubin, porphyrins)
- Uric acid
- Calcification (dystrophic, metastatic, calcinosis cutis)
- Melanin
- Exogenous pigments
- Lipofuscin
- Amyloid
A. Pigments:
- 1- hematogenous pigments (hemoglobin, hemosiderin, bilirubin, porphyrins)
- 4- melanin
- 5- exogenous pigments
- 6- lipofuscin
B. Tissue deposits:
- 2- uric acid
- 3- calcification (dystrophic, metastatic, calcinosis cutis)
- 7- amyloid
You are called out to a sheep farm with increased mortality and you notice several ewes are weak and have pale yellow mucous membranes. What is the term for yellow discoloration of a tissue?
- Gout
- Icterus
- Xanthosis
- Jaundice

- Icterus and 4. Jaundice are both increased bilirubin in tissues

grossly: yellow-green discoloration of tissue or fluid, most prominent in mucous membranes, adventicial surfaces (do not use fat to assess, especially in livestock because they store carotenoids there; sclera of eyes and intima of vessels a good place to look)
What is causing the yellow color in jaundice/icterus?
- Hemosiderin
- Bilirubin
- Hemoglobin
- Haptoglobin
- Melanin
TEST QUESTION
- Bilirubin
* (break down of RBC > macrophages break down hemoglobin into heme, globin, and iron > heme breaks down further into bilirubin)*

Which enzymes break down heme (from hemoglobin) into bilirubin?
(pick 2)
- Biliverdin reductase
- Heme oxygenase
- Biliverdin oxygenase
- Heme reductase
- Biliverdin reductase and 2. Heme oxygenase

EXCEPT birds do not have biliverdin reductase, so the end product is biliverdin
Once unconjugated bilirubin gets into circulation, hepatocytes will take it up and conjugate it with _______________ so it can be secreted into bile.
Diglucuronide

What is too much bilirubin in the blood?
Hyperbilirubinemia
If it gets > 2mg/dL, you get jaundice (it can be conjugated or unconjugated, it will have the same color)

What are the 3 ways that jaundice can occur?
TEST QUESTION
- Prehepatic hyperbilirubinemia: unconjugated bilirubin production exceeds hepatocellular uptake; caused by hemolysis (intravascular or extravascular) [WHAT DIAGNOSTIC TEST WOULD YOU USE? PCV]
- Hepatic hyperbilirubinemia: hepatocellular disfunction (decreased bilirubin uptake, decreased conjugation, decreased secretion in bile) = build up of conjugated and unconjugated bilirubin in blood; caused by hepatic insufficiency, hepatitis, hepatocellular degeneration; severe liver disease
- Posthepatic hyperbilirubinemia: reflux of conjuaged bilirubin into blood; caused by biliary obstruction (cholestasis) or rupture
KNOW THIS
What will you see microscopically in a patient with jaundice?
- Do not see pigment in jaundiced tissues!
- Exception: severe cholestatic (obstructive bile flow) liver
- Yellow-brown intracellular (hepatocytes, kupffer cells) or extracellular pigement (bile canalliculi)

Decreased bilirubin uptake by hepatocytes is
- Prehepatic hyperbilirubinemia
- Hepatic hyperbilirubinemia
- Posthepatic hyperbilirubinemia
- Hepatic hyperbilirubinemia: hepatocellular disfunction (decreased bilirubin uptake, decreased conjugation, decreased secretion in bile); caused by hepatic insufficiency, hepatitis, hepatocellular degeneration

What mechanism of jaundice is to blame with this sheep with widespread jaundice, black kidneys, red-colored urine, and a normal liver?
- Prehepatic hyperbilirubinemia
- Hepatic hyperbilirubinemia
- Posthepatic hyperbilirubinemia

- Prehepatic hyperbilirubinemia
Urine is red from hemoglobinuria (red-brown coloration of kidney and urine, pink serum); microscopically, homogenous red-orange material in renal tubules
How can you tell if hemoglobinuria is from RBCs, hemoglobin, or myoglobin?
Serum

Hemoglobin binds to haptoglobin to be carried in serum = pink serum; myoglobin does not = clear
How does hemoglobinuria look microscopically?
Homogenous red-orange material in renal tubules

In what type of hemolysis will you see hemoglobinuria?
- Intravascular hemolysis
- Extravascular hemolysis
- Intravascular hemolysis (RBC lysis in blood vessels > haptoglobin saturated > Hb free in blood > Hb freely filtered by kidney )

(extravascular hemolysis is what we usually see: abnormal RBCs taken out of circulation by phagocytes > Hb not free in blood to be filtered by kidney > no hemoglobinuria; but both can cause jaundice)
What is the etiology of a sheep with gun metal kidneys (black kidneys), widespread jaundice, normal liver, and red colored urine?
TEST QUESTION
Acute copper toxicosis
(sheep are insufficient in metallothionein for safe copper storage in the liver > chronic accumulation of copper in the liver > mild liver damage > acute copper release > oxidative RBC damage > intravascular hemolytic anemia > hemoglobinuria)

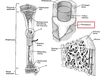
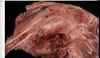
Will we see this change with intravascular hemolysis (intimal surface of a aorta from a horse)?

No, it is a post-mortem change; hemoglobin imbibition
Which is the most severe?
- Prehepatic hyperbilirubinemia
- Hepatic hyperbilirubinemia
- Posthepatic hyperbilirubinemia
- Posthepatic hyperbilirubinemia
(no bilirubin leaving)
Which has the slowest onset?
- Prehepatic hyperbilirubinemia
- Hepatic hyperbilirubinemia
- Posthepatic hyperbilirubinemia
- Hepatic hyperbilirubinemia
Prehepatic, hepatic, or posthepatic hyperbilirubinemia? (dog, generalized jaundice, normal liver)

Prehepatic hyperbilirubinemia (IMHA, usually no intravascular hemolysis)
Prehepatic, hepatic, or posthepatic hyperbilirubinemia? (puppy, generalized jaundice, smaller liver)

Hepatic hyperbilirubinemia (infectious canine hepatitis/adenovirus)
Prehepatic, hepatic, or posthepatic hyperbilirubinemia? (fat cat, intimal surface of aorta is yellow, liver enlarged, pale/tan, greasy)

Hepatic hyperbilirubinemia (hepatic lipidosis)
Prehepatic, hepatic, or posthepatic hyperbilirubinemia? (horse, green-brown liver)

Posthepatic hyperbilirubinemia (biliary calculus)
What would you do with this sick foal to determine the cause of jaundice in this case? (list 4 things you can do clinically)

TEST QUESTION
- Urinalysis for intravascular hemolysis
- Serum chemistry to check bilirubin (liver enzymes)
- Liver imaging (x-ray, U/S)
- CBC (hematocrit or PCV for anemia; if there is no anemia = not prehepatic)
A sick foal was diagnosed with neonatal isoerythrolysis. Its mother (A/Q negative) had given birth to a foal with A/Q blood type with a A/Q positive stallion. The fetal cells passed to the mare’s blood during gestation and she became sensitized. She bred again to the A/Q stallion and the 2nd foal ingested colostrum packed with antibodies against its blood type and resulted in intravascular hemolysis. What other lesions would this foal have?

Red kidneys and hemoglobinuria
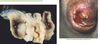
What pigments are responsible for the color of this bruise?

Hemoglobin- red from oxygenized hemoglobin, blue from deoxygenized hemoglobin
Bilirubin- yellow/green
Hemosiderin- tan/brown