Intro, post-mortem morphologies, anomalies, etiologies, cell adaptations and injury, inflammation Flashcards
(353 cards)
Types of pathology studies
structural, biochemical, functional in response to injurious agents and deprivations
General pathology
common reactions of cells and tissues to injurious stimuli
(e.g. acute inflammation in response to bacterial infections)
Systemic pathology
alterations and underlying mechanisms in organ specific diseases
(e.g. liver failure)
Disease
abnormal body process with or without characteristic signs; begins at the molecular and cellular level
Rudolph Virchow
father of modern pathology
Reversible cell injury
injury during the decline of cell function
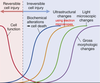
Irreversible cell injury
injury during incline of biochemical alterations (cell death), ultrastructural changes, light microscopic changes, and gross morphologic changes

4 aspects of a disease process that form the core pathology
etiology, pathogenesis, molecular and morphologic changes, and clinical manifestations
Etiology (Etx)
the cause (causative agent, etiologic agent) or origin of a disease or disorder; the study or theory of the factors that cause disease and the method of their introduction to the host
(ex. Canine parvovirus type 2)
What are the 2 major classes of etiology?
genetic/intrinsic (e.g. inherited mutations, disease-associated gene variants, polymorphism) and acquired (extrinsic, infectious, nutritional, chemical, physical)
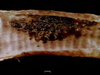
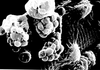
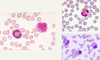
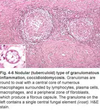
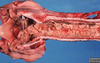
Tissue from a raven; what is the etiology?

Poxvirus
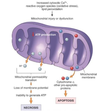
Pathogenesis
the mechanisms of disease development; sequence of events from INITIAL stimulus to the ULTIMATE expression of the disease in the response of cells or tissues to the etiology; always end it with the morphological diagnosis
(ex. oropharyngeal infection > viremia > infection and death of rapidly dividing cells from crypts > necrohemorrhagic enteritis)
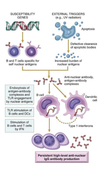
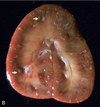
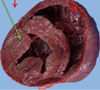
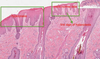
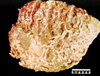
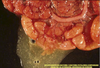
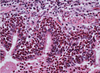
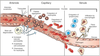
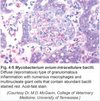
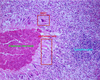
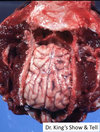
Pathogenesis (img)

Grain overload, rumenal acidosis, mucosal damage, opportunistic fungal infection, vasculitis, ischemia and mucosal ulceration
Molecular and morphological changes
refers to biochemical and structural alterations induced in the cells and organs of the body; the changes may be characteristic (pathognomic) of a disease or diagnostic of an etiologic process
Clinical manifestations
results of genetic, biochemical and structural changes in cells and tissues; manifests as functional abnormalities such as signs (animals) and symptoms (humans, what the patient feels and tells you); injury to the cells and to the extracellular matrix finally leads to tissue and organ injury
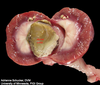
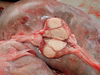
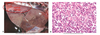
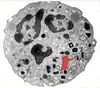
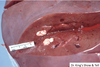
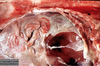
What are the molecular and morphologic changes of the image? ( MDx)
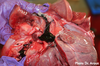
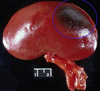
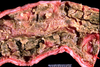
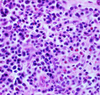
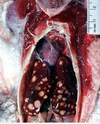
Clinical history and signs: 3 week old puppy, anorexia, dypsnea, abd pain upon palpation, normal rectal temperature

Multifocal, acute renal necrosis and hemorrhage or necrohemorrhagic nephritis
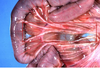
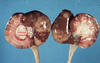
What is the etiology?
Clinical history and signs: 3 week old puppy, anorexia, dypsnea, abd pain upon palpation, normal rectal temperature

Canine herpesvirus-1 (CHV-1)
- red spots are from the virus targeting endothelium
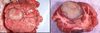
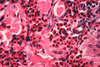
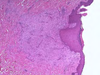
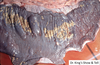
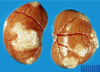
What is the pathogenesis?
Clinical history and signs: 3 week old puppy, anorexia, dypsnea, abd pain upon palpation, normal rectal temperature
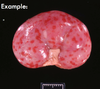
Transmission CHV-1 to pup at birth >incubation period of up to 1 week > virus replicates at temperature lower than 37C (98.6F) > endothelial cell tropism > multifocal necrosis in numerous organs
Diagnosis
concise statement or conclusion concerning the nature, cause, or name of a disease
Types of diagnosis
clinical diagnoses, differential diagnoses (DDx), morphologic diagnoses (MDx), etiologic diagnoses (Edx, agent and organ), clinical pathologic diagnosis
Clinical diagnosis
based on case history (Hx), clinical signs, physical examination; may provide differential diagnosis
Differential diagnosis (DDx)
list of diseases that could account for the evidence or lesions of the case; DAMNITV scheme; clinical or morphological
(ex. clinical: anticoagulant rodenticides, canine distemper, hemorrhagic gastroenteritis due to Clostridium spp.)
Morphologic diagnosis (MDx)
based on the predominant lesion(s) in the tissue(s); macroscopic, microscopic; summary of the lesion, but generally does not describe what is causing the lesion
- location (organ tissue)
- distribution (focal, multifocal, locally extensive, diffuse)
- severity (mild, moderate, severe)
- duration/time (acute, chronic)
- nature (degenerative, inflammatory, neoplastic) of the lesion; if inflammatory remember to classify the type of exudate
(ex. [small intestine], segmental necrohemorrhagic enteritis)
Main types of pathological processes
degeneration/necrosis, inflammation & repair, tissue deposits/pigmentations, circulatory disorders, disorders of growth (adaptation vs. neoplasia vs. malformations)