Physiology of Infection Flashcards
Summarise the characteristics of the different types of pathogens (Viruses, bacteria, fungi and parasites)
x

What are the components of bacteria?
Cytoplasmic membrane, cell wall, capsule, pili, flagella (movement), intracellular structures (nuceloid, ribosomes, inclusion granules, endospores)
What is the function of the cytoplasmic membrane in bacteria?
Made of proteins and PL, no sterols, synthesise and export cell wall components, respiration, secretion of EC enzymes and toxins, uptake of nutrients by active transport mechanisms
What is the function of the cell wall in bacteria?
Protective = rigid, so can withstand osmotic and mechanical stress, provides barrier against certain toxic chemical and biological agents; gives shape to bacterium; firm base for pilli, fimbriae and flagella; contains Ag which are used in virulence and host Ab production
What are the 2 types of cell wall?
Gram Positive -> 2 layers, THICK Ptg/Teichoic acids and inner plasma membrane; Negative -> 3 layers, THIN ptg layer and outer plasma membrane with LPS and outer membrane proteins
What is the function of the capsule of the bacteria?
Mucoid polysaccharide layer, consisting of polymerised D-glutamic acid; capsular Ag; anti-phagocytic activity and prevents attack by complement; adhesion; lab diagnostic tests; vaccines
What is the function of pilli in bacteria?
Sex pilli (transfer DNa by conjugation) and common pilli (attachment) -> adhesion and anti-phagocytic activity
What are the nutrients needed for bacterial growth?
C, N, H2O and source of energy; some need specific gases and growth factors; media developed to ID specific bacteria
What are the classifying features of bacteria?
Shape -> round, long curved, pairs, clusters, chains; Atmospheric requirement -> needs O2, tolerates O2, needs increased CO2 , O2 toxic; Spore production -> dangerous feature
Which tests can be used to identify bacteria?
Enzyme production -> Certain enzymes like urease, catalase, coagulase, oxidase; Toxin production -> protein synthesis inhibitors, haemolysins, superAgs; gram stain -> methyl violet and Lugol’s iodine followed by acetone then methyl red = GPB are violet/blue and GNB are red
How are Gram positive bacteria classified?
Based on shape the biochem test results and aggregation

How are GPB cocci further classified?
Staph differentiated based on coagulase test; strep by extent of haemolysis -> alpha further differentiated by optochin (toxic chemical), beta is Group A (S. pyogenes), B (S. agalacticae) and D (Enterococci); non-haemolytic = strep milleri group and anaerobic strep

Which infections are caused by staph?
S. aureus = Severe infections (skin/soft tissue, endocarditis and osteomyelitis); Coagulaste negative staph = skin commensals of low path potential, infest prosthetic material causing line/pacemaker infections and endocarditis
Which infections are caused by strep?
Group B = neonatal infection as 1/3 of women have group B strep in vagina; pneumonia by S. pneumoniae (found in airs on blood film and optochin sensitive)
What are the types of Gram positive rods?
Spores are dangerous feature -> 2 groups: Bacillus sp (B. cereus and B. anthracis) and Clostridium sp. (C. perfringens/tetani/botulinum/difficile)

What are endospores?
Contain bacterial DNA, cytoplasm, plasma membrane, ptg, very little water, dipicolinic acid and keratin-like coat; formed in response to adverse env conditions, resistant to chemical inactivation; function is protective, so survives drying, heat, dehydration and radiation
What is important about anthrax?
Chemical weapon -> prevented/treated by ciprofloxacin; causes Hide Porter’s disease, which is due to contaminated animal hides. Presents with black eschars, oedema and swelling
What are the different types of GNB?
NB: Listeria is a GPB.

What is present in the periplasm of GNB?
Hydrolytic enzymes, Antibiotic inactivator enzymes, oligosaccharides (osmotic pressure buffers)
What is Haemophilus influenza?
GN cocco-bacilli; found in nasal cavity and doesn’t cause much of a problem because most people are vaccinated against it
What is present in the outer membrane of GNB?
PL, LPS -> Toxic to humans, causes fever and endotoxic shock, consists of Lipid A (toxic part of endotoxin), core polysaccharide, O Ag
Which bacteria can be differentiated using oxidase test?
Oxidase -ve = Coliforms like E. coli, Klebsiella spp., Enterobacter spp., Seratia spp., Proteus spp. Oxidase +ve = Pseudomonas spp.
How do bacteria hang on?
Non-specific electrostatic interactions. Tethering via projections (fimbriae) or pili. Attachment via special receptors on human cell surfaces (e.g. fibronectin binding proteins). Internalisation into the epithelial cell
How do bacteria penetrate the epi/endothelial barriers?
Artificial penetration (breach of epithelium), entry into and through the cell, transit in between cell layers (S. aureus has exfoliative toxin that cleaves desmosomes leading to scalded skin syndrome
What is the innate immune response?
Recognition of pathogen components cause transcriptional changes that lead to cytokine production (TNFa and IL-1); NO is released during infection, leading to relaxation of blood vessels and inflammation

What are Toll-like Receptors?
Lipoproteins from bacteria detected by TLR2 (heterodimer with TLR1/6);
TLR4 detects endotoxins (LPS) for GNB infections;
TLR5 detects flagellin (some GNB) -> activation leads to signalling inside cell and transcriptional changes;
other PRRs are inside cells = NOD and other TLRs

What is the response to meningococcal infection?
Once PRRs activated they trigger release of inflammatory mediators and recruit innate immune cells. NB: many neutrophils in CSF, which is responsible for stiff neck in meningitis; complement is v. important in dealing with meningitis

How are GNB cleared from the body?
Ab opsonise the bacterium before phagocytosis; complements also opsonise; chemotaxis of neutrophils mediated by C5a. MAC (from complement activation) lyses some GNB; IgG (produced by vaccination)
How are GPB cleared from the body?
Don’t have LPS; complement can’t lyse GPB; must be phagocytosed -> IS triggered by ptg (TLR2 and NOD IC) and lipoteichoic acid. Bacterial DNA acts through TLR9. They are cleared via specific Ab opsonising, complement opsonising, chemotaxis of neurophils, clearance by phagocytosis then killing
Which pathogens trigger multiple PRRs and which PRRs do they trigger?
x

What is gram positive Toxic shock?
S. aureus produces a superAg TSST-1;
superAg produce a more profound immune response ->
deceive the IS as they bind to MHC II outside of the Ag binding groove so around 20% of T cells see the sAg and think they are complementary to it, so proliferate ->
MASSIVE proliferation of T cells and production of cytokines, leading to TSS. Ab can neutralise the toxins

How does Strep pyogenes evade the immune system?
Anti-neutrophil strategies e.g. streptolysin Anti-opsonisation strategies e.g. complement binding Toxins that interfere with immune responses e.g. superantigens
How does Staph aureus evade the immune system?
Anti-neutrophil strategies e.g. PANTON VALENTINE LEUKOCIDIN Anti-opsonisation strategies e.g. Ig binding proteins Toxins that interfere with immune responses e.g. superantigens
How does N. meningitidis evade the immune system?
Anti-complement/Anti-MAC strategies e.g. capsule
What is Panton Valentine Leukocidin?
mecA gene confers resistance to beta-lactams; these strains found in hospitals but there are clones in the community that have acquired similar Abx resistance element and a phage that encodes PVL ->Toxin forms a heptamer (heptameric pore) which at low conc = makes human neutrophils apoptose, at high conc = makes human neutrophils lyse -> very nasty infections associated with abcesses that can’t be cleared by the pts
What is the function of innate immunity?
Prevents infection and can eliminate infection with/without interaction with acquired IR; early response to infection involving phagocytic cells; drives adaptive IR; no memory; recognises microbial conserved structures that aren’t found in host (PAMPs); no self reaction; targets products needed for microbial survival; germ line components
What are the components of innate immunity?
Physical barriers - skin, mucociliary escalator; cellular barrier (immune active); circulating effector leukocytes (monocytes/macrophages/neuts/NK cells); circulating proteins (complement, collectins and pentraxins (CRP), antimicrobial peptides (defensins)); commensal organisms (gut microbiome); cytokines; local enzymes
What are NK cells?
Specialised T cells, stim by IL-12 and IL-15; detect missing self and kill infected/malignant cells; secrete cytokines (IFN-gamma) which activate macrophages, activate receptors and signal via tyrosine kinase receptors (Syk and Zap70), inhibitory receptors have immunoreceptor tyrosine inhibitory motifs in cytoplasmic tail
What is phagocytosis and how does it work?
Cells -.> macrophages, monocytes and neutrophils, which have PRRs that recognise PAMPs; mannose receptors bind mannose and fucose receptors on microbial GP/lipids. Scavenger receptors = CD14 (no cytoplasmic tail; opsonisation by complement (C3!!) and Abs then ingestion into vesicles. Macrophages can disseminate mycobacterial infection
What are TLRs?
Give specificity to innate IS; recognise and discriminate specific microbial components. TLRs interact with various adapter molecules = CD14 and MyD88; activate proinflammatory cascade; act IC/EC
What are the types of cytokines?
Proinflammatory -> lead to release of many other cyto/chemokines = TNFa and IL-1; Regulatory -> activates adaptive immunity = IFNg and IL-12; Down-reg -> IL-10 and TGF-b; Chemoattractants -> Chemokines
What are cytokines?
Secretion is usually transcriptionally regulated and transient; para/endo/autocrine, systemic/local; bind to specific receptors; effector functions are multiple and cell specific, may act synergistically/antagonistically
What are the 4 main properties of cytokines?
Pleiotropism, synergism, antagonism and redundancy
What is innate immunity the balance of?
Destructive: Proinflammatory damage, hgih TNF, IL-1 ^^^ and MMPs; VS. Protective low TNF, IL-1 ^, IL-10 and TGF
What is TNFa?
Major source is mononuclear phagocytes; secreted early in infection; apex of cascade of proinflammatory cytokines; mediates LPS response; causes cachexia and septic shock; affects muscle function (depresses heart); drives met disturbance = decrease blood glucose and emerging interaction between met and immunity
What are TNF receptors?
2 main types that TNFa binds to -> p75 TNF and p55 TNF;
CD40 is also an important member of the TNFR family. CD40 member of TNF family;
some TNF receptors have DD which activates death signalling pathways involving caspases leads to cell death;
TNF can cause necrosis and apoptosis

What are NFkB transcription factors and how does the signalling pathway work?
Sits in cytoplasm as heterodimer of p65 and p50 bound to IkB; when athogen triggers IC signalling via receptors, IkB is phosphorylated, ubiquinated and degraded, allowing NFkB to pass from cytoplasm to nucleus, where it binds to binding sites which are nearly on all pro-inflammatory genes, with binding occurring in matter of minutes and is essential for some cyto/chemokines. NFkB and IkB have different forms; p65 alone is activating; p50 and p52 alone or with other molecule are inhibitory. IkBa is classical; IkBb binds to NFkB and keeps it in active state, so maintains NFkB as an active molecule
How are inflammatory genes regulated by transcriptional control?
TF binding sites near the genes, mRNA has regulatory sites (UA rich regions at 3’ end, providing stability) = more UA rich sequences means more proteins produced
How is IFNg important?
IFN -> IFNR -> phosphorylation of JAKs -> activation of STAT1 -> heterodimerisation to activate genes in nucleus. IFNg deficient pts are very susceptible to mycobacterial infections and salmonella -> can be caused due to deletions of IFNR, acquired AIAb.
Lesser clinical variants have IL-12 system mutations

What are chemokines?
They recruit leucocytes to areas of infection and inflammation;
important in angiogenesis. 2 groups (has 4 cysteine molecules but proximal 2 give name) = CC, usually recruit mononuclear cells (monocytes and lymphocytes = MCP-1 and RANTES) (2 adj cysteine residues) and CXC usually neutrophil attractants (IL-8 = neutrophil attractant with ELR leader sequence;
IP-10 = T cell attractant) (2 cysteine residues separated by another amino acid);
small family have just one cysteine residue = lymphotactin.
Can either be inducible or consitutively expressed

How are leucocytes recruited?
Roll along endothelial wall and if activated then binds to selectin molecules (loose binding) and then more tightly via integrins which transmigrate through the cells into the tissues

What is the role of IL-8 in sepsis?
Released by neutrophils and monocytes, present in animal models of sepsis, with raised IL-8 associated with poor prognosis; Ab protect in lung reperfusion injury
What is haplotaxis and how are chemokines involved in chemotaxis and adhesion?
Many chemokines attached to tissues but at different concentrations and the cells migrate down tissue-bound gradient; - anchored chemokines are potential adhesion molecules = chemokine bound to a cell or GAG and sits acting like adhesion molecule for cells; IL-8 upregs beta2 integrins. Chemokine receptor is GPCR (7-TMR)
How are chemokines involved in molecular mimicry?
Lot of chemokines mimicked by pathogens; HSV = ECRF3 which mimics IL8Rbeta; CMV US28 mimic ubiquitous chemokine receptor that bins MIP-1alphaR, RANTES and MCP-1
Why do viruses express chemokine receptors?
More chemokines = more spreading from one cell to next; expressing chemokine receptors on surface and acts as a sponge that soaks up chemokines and stops leukocytes being recruited to site of infection
What are the complement pathways and their effects?
Alternative (microbe activated), classical (Ab activated), lectin (lectin activated - MBL) -> early effects: inflammation (C3a), opsonisation and phagocytosis (C3b); late effects:inflammation (C5a), lysis of microbes (MAC)
What are MMPs?
Breaks down collagen; family of zinc containing proteases, classified by substrate specificity (gelatinases, collagenases, stromelysins, MT-MMPs); key in tissue remodelling and embryonic development; have important immunological functions = leukocyte migration (breaks down tissue to carve pathway for leukocytes to migrate), activate/deactivate range of cytokines and chemokines; excessive MMP is destructive
How are MMPs involved in TB?
MMPs involved in leucocyte recruitment to sites of infection;
drive tissue destruction in TB (matrix degrading phenotype, required for spread of infection (pulm cavitation creating immune-privileged site).
acts in networks - granulomas in TB, MMPs as potential therapeutic targets -> TB infection in monocyte leads to: MMP-1 = Type 1,2,3 collagen (critical for tissue destruction) and MMP-9 = Type 4 collagen.
Shift to matrix degradation in TB;
Cavitation key feature in TB which results from upreg of protease activity leading to lung EC matrix destruction
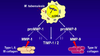
What are the mechanisms of avoiding innate immunity?
x

How does the innate immunity kill microbes?
x

What determines pathogenicity of a commensal?
Partly on compartment that they are found in
What are the 4 different types of resolution of infections?
RSV immuno memory wanes over time, so reinfection can occur by same pathogen.
For HIV there is acute phase but then virus suppressed by body but not totally cleared and viral load builds up slowly over time.
CMV = no acute phase, gradual build up of amount of virus in body over time

Why do some diseases spread?
2 main variables -> Basic reproduction number (how many people does one virus infect) an proportion of infections that occur PRIOR to symptoms (symptomatic before being infectious then quarantine subjects to prevent spread)

What are the variable factors in susceptibility/host response to infection?
General health and nutritional status; previous exposure; immune defeciencies (SCID, HIV), polymorphisms in innate immune genes; polymorphisms in adaptive immune genes (HLA)
What are the 4 main routes of infection and the type of organisms that use each route?
NB: Resp infections are the most common type; GIT is also common site of infection

How can you prevent infection at mucosal surfaces?
Mechanical - epithelial tight junctions and longitudinal flow of air or liquid; Chemical - fatty acids, enzymes, low pH, Abx peptides, mucus; Microbiological - Normal flora compete for nutrients/attachment sites, production of antibacterial substances
How is mucus useful for preventing infection?
Produced by goblet cells, contain mucin glycoproteins; traps dust and pathogens which are swept upwards
How are proteolytic enzymes useful for preventing infection?
Produced in stomach (pepsin) and small bowel (trypsin); breakdown large polpeptides into di/tripeptides; small peptides are poor immunogens; enzymes are cytotoxic to pathogens
What are the types of antimicrobial molecules and how are they useful for preventing infection?
Lactoferrin = binds iron and inhibits bacterial growth; Lysozyme = cleaves cell wall of GPB; Defensins = 30-40 a.a. peptide that disrupts the cell membranes of bacteria and fungi causing lysis
How are commensal organisms useful for preventing infection?
Compete with pathogenic bacteria for space and nutrients; prevent colonisation of gut by pathogenic bacteria; commensals important in GUT and resp tract; ABx disrupt immune homeostasis by killing commensal microbes that compete with pathogens -> faecal transplants involve killing commensals of GIT and repopulating with transplanted faeces
How is skin useful for preventing infection?
Physical barrier; virtually no invasion via this route; skin is colonised by potentially pathogenic bacteria, with many pathogens causing disease if barrier broken
How does the respiratory system react to bacteria?
URT is colonised - transient bacterial popn can cause disease; fine hairs and nasal membranes can trap bacteria. LRT is protected well (also colonised) by mucosal surfaces, lysozymes, sweeping movement upwards by ciliated epithelium, antimicrobial peptides and B-defensins made by epithelia, alveolar macrophages also involved in defence
How does the digestive system react to bacteria?
Colonised by large numbers of MOs but transient popn can cause disease; Oral = compete with adapted normal flora of mouth and intestine; stomach = acid and secretions; lower intestine = alkaline pH; intestine = peristaltic action flushes out the organisms; pancreatic enzymes = bile salts, lysozyme. NB: pH transition can kill many bacteria as some may survive the acid but not the alkali
How does the Genitourinary system react to bacteria?
Male = sterile, female colonised; urine used as flushing mechanisms; acidity of urine and vaginal epithelium (colonised by lactobacillus acidophillus producing an acidic end product preventing colonisation)
How does the immune response work?
Pathogens invade the epithelium and triggers the innate IS (is specific but recognises a smaller family of molecules than adaptive IS;
innate IR leads to Ag release which leads to bridging between the 2 arms of the immune response;
Ag are taken up by APC and shown to T cells on MHC

What are the 3 main functions of the innate immune system?
Recognition - picks up on infection in first place; releases inflammatory mediators to recruit other cells to the area; effector function - some of the innate cells are effector cells and can kill other cells
What are the 3 phases of the immune response?
The immediate phase is characterised by release of preformed molecules

What are the 4 pathogen control strategies and pathogen sensing in vivo?
The 4 strategies = Prevention of pathogen entry; Destruction of pathogen prior to cellular entry; Recognition and destruction of infected cells; Inhibition of pathogen products (blocks the toxins produced by viruses). Pathogen sensing in vivo is where resident cells detect pathogens (epithelial cells, resident mast cells, macrophages, DC), with infection detected by PRRs
What is the TLR pattern recognition pathway?
TLR is PRR; recognise PAMPs and each TLR recognises different PAMP = TLR2 - lipopeptide, TLR4 - LPS, TLR9 - CpG repeats. Pattern recognition results in IR becoming turned on, which leads to -> release of inflammatory mediators, initiation of acute inflammatory response and recruitment/activation/maturation of effector cells
What are neutrophils?
Early response in acute response; preformed, non-specific responders -> release toxic granules and engulf pathogens. They are recruited by chemokines, primed by cytokines, activated by pathogen contact. Have anti-bacterial function = mainly BACTERIAL
What are NK cells?
Cytotoxic cells important in killing of infected cells and tumour cells; 2 main functions in infection = lysis of target cells, secretion of cytokines; mechanisms of killing cells = granzyme/perforin and FasL/Fas pathways. Mainly involved in VIRAL infections
What are macrophages?
Engulf foreign particles which are often tagged by Ab (opsonisation). Activated by cytokines (IFNg); kill bacteria in phagolysosome using respiratory burst (reactive O2 burst release). Present Ag on MHC I/II
What are the actions of macrophages and DC?
Both are APC; activated by innate immune system stimuli - TLR ligands; acts as a bridge between innate and adaptive immunity; activate T cells. Macrophages release cytokines that cause fever
How is the MHC class I loaded?
Virally infected cells produce protein ad some of these proteins will be misfolded - misfolded proteins tagged by ubiquitin and driven into the proteosome which processes the protein and produces smaller peptides;
peptides get pumped through TAP and loaded onto MHC I which translocates to the cell membrane
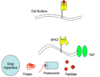
How is MHC class II loaded?
MHC II presents external proteins, with 2 IC bodies involved = MHC containing lysosome and phagolysosome containing phagocytosed proteins.
External proteins are taken up into phagolysosome and get chewed up into smaller peptides.
2 IC bodies merge and peptide loaded onto MHC class II by HLA-DM;
loaded MHC II then moves to cell membrane

Summarise the Ag presentation pathways to CD8 and CD4 cells
x

What are CD4+ T helper cells?
CD4 cells amplify the IR by recruiting and activating other cell types by producing cytokines

What are CD8+ T cells?
CTL; kill by releasing granzymes and perforin or by engagement of Fas on target cells by FasL; also produce IFNg and TNFa; responsible for exerting a considerable selective pressure on virus altering CTL epitopes. CTLs can recognise non-self when MHC I presents non-self peptides. NK cells detect missing self underexpression of MHC I as a result of virus infection
What are B cells?
Express cognate receptors on surface; turn into plasma cells to produce Ab; can be either T cell dependent (need CD4+ help) and T cell independent (highly repetitive polysaccharide Ag); important to prevent future reinfection; some role in clearance of pathogens but main role is Ab
What are Ab?
Lyse membrane bound virus particles; opsonise particles (enhances phagocytosis via Fc receptors on immune cells/via complement; lyse infected cells or pathogens; triggering of inflammation and immune cells; function often involve complement; Ab neutralise bacterial toxins, can bind to epitopes on the pathogen and prevent entry -> exploited in passive immunity
How is the pathogen destroyed before entry?
Membrane Attack Complex -> complement-dependent damage to cell membranes; works with bacteria if the membrane can be targeted; works with enveloped viruses
What is Ab dependent cytotoxicity?
Mediated by NK cells; Ab bind to epitopes on the virus-infected cell; NK cells binds to the Fc portion of the Ab; results in NK cell mediated lysis of the infected cell
How do vaccinations help acquired immunity?
Vaccine particles are engulfed by APCs digested into peptides and presented to T cells, which help B cells make Abs, so vaccines mimic normal IR to pathogen, with 2nd exposure Ab response is much stronger and faster than 1st time round
What is passive immune evasion?
Antigenic drift and shift leading to epitope escape -> drift = small changes in Ag over time and still look quite similar; shift = viruses come from an animal source and it looks totally different to anything previously encountered by humans; anatomical seclusion/hiding (in immune privileged sites); infection of the immune cells themselves
What is the active immune invasion?
Bacterial/viral proteins can block various steps within the innate and acquired IR

How does HIV evade the immune response?
They target CD4+ T cells;
HIV also infect via APC;
HIV particles have coat proteins that are covered by carb to evade recognition;
epitopes in gp41 are masked (important in viral entry);
RNA genome undergoes rapid change both CTL and Ab are evaded - high mutation rate;
vaccination increases CD4 T cell infiltrate to the infected area, therefore increasing target cells for the virus

Summarise the factors that affect infection outcome after exposure to the microbe
x
What is purpura fulminans?
Bleeding into the skin and most commonly caused by meningococcal septicaemia; obvious appearance of what sepsiis looks like
What are Koch’s postulates?
Characteristics of things that cause disease; they state that to organism causing disease must be: Found in all cases of the disease examined; Prepared and maintained in a pure culture; Capable of producing the original infection, even after several generations in culture; Be retrievable from an inoculated animal and cultured again
What is sepsis?
NOT a disease; it is a series of pathological processes that make people very ill and can be caused by a number of different routes. Sepsis is SIRS with presumed/confirmed infectious process
What is SIRS?
Systemic inflammatory response syndrome - non-specific clinical response inc. >2 of: Temp more than 38, less than 36; HR >90bpm; RR >20/min; WCC >12,000/mm3 or <4000/mm3 or >10% immature neutrophils; SIRS can also be caused by trauma, burns, pancreatitis and other insults
What is severe sepsis?
Sepsis with signs of at least one acute organ dysfunction: renal, rep, hepatic, haematological; CNS; unexplained metabolic acidosis; cardiovascular - most important organ affected is vascular epithelium
What is Septic shock?
Severe sepsis with hypotension despite fluid to restore adequate volume
What is SOFA?
Sepsis-related Tr organ failure assessment -> allows you to measure how deranged peoples organ functions are
What are the pathogenetic mechanisms of gram negative sepsis?
Tend to be in gut; LPS is a key factor associated with GNB toxicity - more endotoxin = worse chances of survival , but not that infection is worse; in shock you have a higher chance of having endotoxin in circulation (if blood vol decreases due to blood loss, then blood doesn’t circulate to gut so well; GIT has GNB which if lack of perfusion in gut, leads to breakdown of gut mucosa so endotoxin enters circulation, which usually the liver can handle, but immune system gets overwhelmed and endotoxin enters systemic circulation
What does LPS consist of?
Lipid A (toxic component - direct effect: triggers complement, coagulation, fribrinolytic and kinin pathway; indirect: initiator of cytokine cascade in inflammatory response), core region and O-Ag polysaccharide
What are the important nosocomial infections?
Enterecoccus, Staph aureus, C. difficile, Acetineobacter baumanii, Pseudomonas aeruginosa, E. coli (enterobacter spp.); with nosocomial pneumonia as most important
How does the pt progress to sepsis and severe sepsis?
- Endotoxin binds to LBP in circulation, with E-LBP complex binding to CD14 receptor on macrophages/monocytes,
- sending a message to the genome of the cell so it reacts in a particular way - CD14 doesn’t have transmembrane domain,
- so can’t directly talk to inside of cell, so binds to TLR4,
- which talks to the genome leading to transcriptional changes in the cell.
- Neutrophils are large cells which can get stuck and end up in alveoli, releasing damaging cytokines, causing leaky vascular endothelium so oedema occurs, also unreg release of NO happens causing vasodilation and drop in BP

What is the role of the endothelium?
Interacts with leukocytes; release of cytokines and inflammatory mediators; release of mediators of vasodilation and vasoconstriction; functional effects on the coagulation system
What causes cardiovascular failure in sepsis and how do you support it?
Hypovolaemia = disease, leak, reduced vascular tone; hypotension = hypovolaemia, reduced vascular tone, myocardial depression; shock = failure oxygen supply and utilisation. Support = maintenance of circulating volume, give fluid and restore BP using vasoactive drugs (A/NA, dobutamine, VP)
What causes renal failure in sepsis and how do you support it?
Hypovolaemia, hypotension, intrinsic vasoconstriction, acute tubular necrosis; Support = vol resus, BP resus, diuretics, renal replacement therapy (dialysis)
How do you manage sepsis?
Recognise pts at risk, resus, call for help, provisional Dx, investigation and sampling, early Abx, source control -> measure lactate within 3h of potential sepsis. Sepsis Six -> admin high-flow O2, take blood cultures and consider infective source, admin IV ABx, admin IV fluid resus, check Hb and serial lactates, commence hourly urine output measurement
How does C. difficile infiltrate and what are the main symptoms?
Usually occurs following Abx Tx for something else, wiping out commensal organisms. Symptoms = Fever, diarrhoea and later abdominal pain
What are the virulence factors of C. difficile and how do you treat it?
Toxin A, B and Endospores. Toxin A/B = disrupt tight junctions, inactivate Rho GTPases, epithelial permeability increases, release chemokines, neutrophil infiltration, mast cell activation. Tx: stop other ABx, metronidazole (up to 400mg/8hr for up to 10d) or Vancomycin (125mg/6h) PO, consider IVIg, IV fluids
Which disease syndromes are caused by severe C. difficile infection and why is one in particular important?
Pseudomembranous colitis, perforated colon (major consequence is faecal peritonitis which can lead to endotoxin release and septic shock -> TLR4 signalling leading to activation of inflammatory response, endothelial activation, unreg NO production -> hypoperfusion and acidosis => ORGAN FAILURE), dehydration, fluid/salt imbalance, pH imbalance, skin breakdown/pressure sores.
How can you predict C. difficile colitis?
Over 70y; previous C difficile infection, use of anti-peristaltic drugs; Girotra’s Triad: Increasing Abdo pain/distention and diarrhoea, leukocytosis (>18,000), haemodynamic instability
What is the risk ratio?
Observed number affected people in affected cases/ expected number of affected people
What is an example of single gene disorder?
Cystic fibrosis
What is an example of polygenic disease?
Multiple sclerosis, IDDM, Rheumatoid arthritis
What are some sites for genetic influence?
Nasopharyngeal colonisation, in the bloodstream (IR), survival in blood (asymptomatic bacteraemia, meningitis, septic shock, purpura fulminans
Which genes can determine susceptibility vs severity?
Susceptibility(present with greater frequency in cases): control colonisation invasion and survival in blood; severity (same freq in cases, preferentially in severe/fatal cases): determine inflammatory response, extent of coagulopathy, organ failure
How do you identify the genes for a disease?
Candidate gene approach, linkage analysis, genome-wide association, next gen sequencing
What is homozygosity by descent?
Rare recessive defect introduced into a popn by a common ancestor, affected patients will be homozygous, not only for the abnormal gene, but also for those in the vicinity of the defect
What is the IFN gamma receptor?
Expressed on all cell, type 2 cytokine receptor, gene on chromosome 6q; increases susceptibility to salmonella and mycobacterium
What is the CARD11 gene?
Increases susceptbility to chicken pox, leading to death Not familial; new mutation
What are the 2 genetic causes of meningococcal disease?
Familial (>2 cases) = consanguinity; extreme phenotype = death, amputation, skin graft, vaccine failure, severe. Gene mutations: BPIFA1, IL1RL1, complement, JAK2, NEMO
What is complement factor H?
Binds to meningococcal protein, regulating complement -> inhibitor of complement system. Meningococcus has on its surface human factor h, which coats the bacteria, so complement can’t work (trojan horse). Also present on endothelial cells, which could account for the rash. FHR3 regulates amount of factor H. NB: s. pyogenes, H. influenzae also uses this method.
What is an assay?
Method of measuring concentration of a hormone in body fluid (serum)
What is a bioassay?
Administer extract of pit to animal and observe -> now use cell in vitro, which grow when the chemical is present
What is an immunoassay?
Ab could bind to particular protein, could be used to measure the amount of substance -> make Ab by giving protein to different species
Example of immunoassay with adrenomedullin:
Assay for human AM; give AM to rabbits until they make Ab (months), collect blood from rabbits and use that blood to mop up Ag
What is radioimmunoassay?
Make one amino acid radioactive, to know how much you have of RA AM. Also, unknown amount in animal not RA AM, which compete for Ab. Add known amount of Ab to mixture, then 2nd Ab against the first, which should be anti-rabbit and bound to solid particle that sinks when centrifuged; mix and spin
What are the 2 types of immunoassays?
Competitive and sandwich assays; both use enzyme specificity
What is a competitive immunoassay?
Peptide given to rabbit, with Ab formed in the rabbit, which in blood will stick Ab to Ag. Then radiolabelled version of peptide is added, with a mouse anti-rabbit Ab which has a carbon attached, so is heavier. Thus normal Ab can attach to either radiolabelled or not equally. After centrifuging you count the bound:free ratio of radiolabelled peptide (work out by graph)
What is serial dilution?
Used to measure known amount of protein - can then use for immunoassays to work it out. Set up series of tubes with known varying concentrations of Peptide and set amount of radiolabelled peptide. To each add amount of Ab, which bind randomly to radiolabelled and non-RL peptide. Then add 2nd Ab. Detect radiation amount from light and heavy regions of mixtures - inverse correlation between bound: free ratio and concentration of peptide
What is immunoradiometric (sandwich) assay?
2 monoclonal Ab to different epitopes of the protein. 1st covalently bound to tube and 2nd is radioactive, specific to other terminal. More expensive and more precise, with positive correlation. Excess solution is washed away
What are the different types of bias?
Selection, prevention, compliance, survivor, prevalence-incidence
What is a SOFA score?
The method of scoring sepsis in ICU, with qSOFA being carried out in GP/non-hospital settings -> if infection is suspected and SOFA is more than 2 OR there’s an increase of SOFA by 2.

What infections are caused by group A strep?
Invasive: affecting all gender/ages, with rapid onset; associated with all strains of S. pyogenes.
Most strains 5+ superAg genes; usually bacteraemic; 20-60% mortality
Tx: ITU, debridement, Abx, antitoxin Abx IVIg

What is spretococcal toxic shock?
Toxic shock = subset of invasive group A strep infections (10%), with 45% mortality rate; necrotising fasciitis associated with 7x increased risk

How does streptococcus get from the throat to tissues?
Skin lesions, skin trauma, intact skin, blunt trauma, no recalled skin lesion/trauma


