Pharmacology 3: Receptors Flashcards
What is a receptor?
A receptor is a protein-molecule that receives chemical-signals from outside a cell to trigger a response or effect. ‘It is a means of communitcation’
Recall the lock and key model:
Keyhole = receptor, key = endogenous agonist, bobby pin = exogenous agonist, stick = antagonist, lock/unlock = effect
What is an Endogenous agonist?
A type of agonist for a particular receptor that is naturally produced by the body
What is an exogenous agonist?
A type of agonist that binds to a receptor of a cell usually designed for endogenous agonists, and triggers a response by that cells. They mimic the action of endogenous agonists.
Receptors are a means of ______?
Cell communication.
- They wait to receive a signal → then activate a chain of communication → changes in gene regulation → changes in the cell.
E.g. Receptors on leukocytes detect inflammatory mediators released in acute inflammation, leading to migration along the chemotactic gradient towards the inflamed area.
- In addition to communication between cells in our body, receptors are also important in communication between us and the outside world.
E.g. Rhodopsin receptors (G protein coupled receptor) in our eye that detect light+taste receptors+olfactory receptors
What are the four main drug targets?
1. Receptors: Generally proteins that interact with endogenous ligand or external chemical
2. Channels: Controlled by changes in membrane potential
3. Enzymes: Proteins that increase the catalytic rate of biological reactions in the body
4. Transporters: Moving things from outside-inside or vice-versa
Where are receptors found?
In the transmembrane, or intracellular or nucleus.
Most receptors are at the cell’s surface so they can detect signals coming from the outside the cell. Nuclear receptor are found in the intracellular part (cytoplasm or nucleus).
What are the four major types of receptors?
1. Ligand-gated ions channels
2. G-Protein coupled receptors
3. Kinase- Linked (CATALYTIC) receptors
4. Nucleur receptors
What three receptors are embedded in the membrane?
- Ligand-gated ions channels
- G-Protein coupled receptors
- Kinase- Linked (CATALYTIC) receptors
What are the features and structure of Ligand-gated ion channels?
- Each peptide chain begins with an N terminus outside the cell (where drugs bind). C terminus is outside the cell
- The peptide crosses about 4 times. Blue bars represent transmembrane helices.
- You need four or five of these individual peptide units to make a channel. These subunits come together to form a pore through which ions can flow (changing membrane potential). These channels are gated, when a ligand binds there is a conformational change in the receptor proteins and the pore opens up.
- Changing the subunits changes the function
Ligands include acetylcholine, ATP, glutamate, glycine, serotonin and GABA

Which type of receptor is this?

What are the features and structure of G-protein coupled receptors?
- Embedded in the plasma membrane. N terminus is extracellular. Drugs either bind to the N terminus or to the middle of the transmembrane helices. C terminus is inside the cell (where signalling proteins bind)
- Always have 7 transmembrane helices (crosses of the membrane) – egg cup shaped, splayed out at the top and bottom. They have 3 intracellular and 3 extracellular loops. It is on one of these intracellular loops that the G-protein binds.
GPCR protein superfamily is one of the largest known protein families. It is encoded by about 3% of the human genome. About 850 different GPCRs in our body. 250 have known ligands, the rest our orphan receptors.
- GPCR are the most abundant drug targets to date.
GPCRs include receptors for dopamine, adenosine and histamine, adrenogernic (that adrenaline works on), opiod
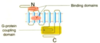
What type of receptor is this?
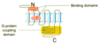
What are the features and structure of Catalytic (kinase-linked) Receptors?
- Embedded in the plasma membrane. Only have one transmembrane helix. N terminus is outside the cell (where the drug binds), C terminus is inside the cell (catalytic domain).
- Catalytic receptors are formed of 2 (sometimes 4) proteins.

What type of receptor is this?

How is insulin binding different to normal binding in catalytic receptors?
The extracellular component is already connected. Insulin binds to the receptor causing a conformational change in the catalytic domain (causing signalling).
All catalytic receptors dimerise when bound to ligand to form the funtional receptors.
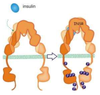
What are the four main types of catalytic receptors?
PAY ATTENTION TO 1 AND 3
- Receptor Tyrosine Kinase (RTK)- Contain intrinsic tyrosine activity (EDG, VEGF, INSULIN)
- Receptor Serine/Threonine Kinase
- Tyrosine-Kinase Associated Receptors (Cytokine receptors)
- Receptor Guanylyl Cyclases
What are the features and structure or Nucleur receptors?
- Do not sit in the plasma membrane but instead float around in the cytoplasm or nucleus. They have a ligand binding domain (carboxyl terminus) that interacts with ligands and a DNA-binding domain (called zinc fingers) that interacts with DNA.
- Ligand crosses the plasma membrane and binds to the receptor. The receptor dimerizes (joins with another receptor) and travels into the nucleus where it binds onto responsive elements of the DNA. Cofactors bind which leads to mRNA that is then translated into proteins. Hence, drugs that target these receptors take a long time to work.
- Class 1: In the cytoplasm and have high affinity for their ligands. Dimers are made of two identical receptors (homodimers). Mainly endocrine. High affinity.
- Class 2 are in the nucleus and have low affinity for their ligands. Dimers are made of RXR and another (different) receptor.
What are the two classes of Nuclear Receptors?
Class 1: In the cytoplasm and have high affinity for their ligands. Dimers are made of two identical receptors (homodimers). Mainly endocrine. High affinity.
Class 2: are in the nucleus and have low affinity for their ligands. Dimers are made of RXR and another (different) receptor.
Which receptors are quickest in order of highest to lowest speed?
Ligand-gated ion channel (milliseconds)
G-protein coupled receptor (seconds)
Kinase-linked receptor (hours)
Nucleur receptors (hours)- Gene transciption and protein synthesis takes forever. Long term chronic inflammation care, USE THIS!!


