Periodicity Flashcards
Ionisation Energy
Ionisation Energy or Ionisation Potential of a species A can be defined as the molar internal energy change, ΔU, for the reaction: A(g) → A+(g) + e-(g) at 0 K, reactants and products being in their standard states. We often assume that ΔH(298 K) ΔU(0 K).
Bond dissociation energy
Bond dissociation energy is defined as the enthalpy change associated with the reaction in which one mole of the bond is homolytically broken, reactants and products being in the ideal gas state at 1 bar and 298.15 K. e.g. for methane, the C-H dissociation energyy refers to: CH4(g) → CH3.(g) + H.(g)
Boiling point
Boiling point of a substance is the temperature at which the vapour pressure of the liquid is equal to the pressure exerted on it by the surroundings.
Melting point
Melting point is the temperature at which the solid and liquid forms of a pure substance can exist in equilibrium.
missing
fill
intro lec until long form
Zeff value across a row
- more protons in nucleus Zeff increases across a row
- more electrons in valence shell Zeff will decrease due to e- repulsion
1 beats 2 so Zeff will increase

Zeff value down a group
Zeff initially increases then remain constant

Slaters rule apply only for
s and p block
Slater’s rules
s is screencing constant = # of e- + shielding contribution depending on the rule
Z atomic number

Ionization energy graph

Ionization energy discontinuation
1) Be and B
2) N and O
- s to p ( p has a node) shielded Zeff lower so IE lower
- Energy K known as exhange energy per pair
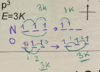
Ionization energy down a group

ionization energy down a group exceptions


