Pediatrics Flashcards
How do you caculate PGA?
Post-gestational age
weeks gestation @ birth +current age in weeks
What is a neonate? Preterm?
Low birth weight?
neonate= birht - 30 days
preterm <37 weeks
low birth weigth <2500 grams
What is extremely low gestational age (ELGAN)
23-27 weeks gestations; all organs immature
most vulnerable peds patient
What are pre-terms at risk for?
- Respiratory distress
- apnea
- hypoglycemia
- electolyte disturbance (particularly hypomagnesemia and hypocalcemia)
- infection
- hyperbilirubinemia
- polycythemia
- thrombocytopenia
Definitions for neonatal period?
Normal gestation? Postmature?
Risks for both age groups?
- Normal gestation 37-42 weeks
- all gestational ages have risk for
- congenital abnormalities,
- viral infection,
- perinatal depression,
- fetal alcohol syndrome
- thrombocytopenia
- all gestational ages have risk for
- Postmature >42 weeks
- risk of meconium aspiration
- birht trauma if large for gestational age (LGA)
- hypoglycemia
- hyperbilirubinemia
- plus above risks for normal gestations.
What is the significance of 60 weeks PGA?
What should you always evaluate?
- Former premature infancts up to 60 weeks PGA are at increased risk for postoperative apnea and braydcardia
- requires postop monitoring, admission, and 12 hour period free of apnea
- Always evaluate perinatal history
- gestation age and size at birth
- maternal infections
- eclampsia
- diabetes
- drug abuse
- prolonged or traumatic labor
- NICU/Intubation following delivery
Why is size at birth important?
- small or large for gestational age babies are more likely to have problems with metabolic, developmental, infectious or structural abnormalities, drug addiction and withdrawl
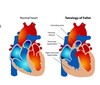
Characteristics of fetal circulation?
- High pulmonary vascular resistance and low systemic circulatory resistance
- minimal intrauterine pulmonary blood flow: only 10% CO
- At birth, placenta is no longer primary source for oxygenated blood
- Basics
- placenta–> umbilical vein–> liver sinusoids and ductus venosus–> IVC–> RA–> foramen ovale (because of pressure, blood shoots across here)–> LA (small amt of mixing with blood from pulmonary veins)–> LV–> ascending aora–> heart, brain UE (most oxygenated blood)–> mixing with deoxygenated blood from ductus arteriosus–> mixed blood feeds thoracic/abd brances–> end of aorta gives 2 umbilical arteries that return blood to placenta
- Blood from SVC mixes with blood in RA–> RV–> 10% goes pulmonary artery to lungs, most blood goes–> ductus arteriosus–> aorta arch that mixes with pre-ductal blood

What are some primary changes that occur in the transition from fetal circulation to birth?
- Ductus venosus closes
- blood is oxygenated via lungs
- ductus arteriosus closes (due to increased arterial O2 concentration)
- pulmonary vascular resistance DECREASES
- peripheral vascular resistance increases
- foramen ovale closes
- true closure weeks later; 25-30% of adults have patent foramen ovale
- only a functional closure at birth
- all these changes can reverse in stressed newborn.

What is transitional ciruclation?
Prevention? Treatmenbt?
- occurs at birth for the first several weeks
- hypoxia, hypercapnia, or hypothermia can lead to increased pulmonary artery pressure, which causes reversal of flow through foramen ovale (meaning returning to fetal circulation), reopening of ductus arteriosus and shunting
- deoxygenated blood perfuses systemic circulation and this hypoxia is difficult to correct
- prevention: optimal oxygenation, correct acidosis, keep warm
- treatment: hyperventilate to reduce PaCO2
Characteristics of newborn heart?
- Newborn heart:
- structurally immature
- fewer myofibirls (not parallel)
- sarcoplasmic reticulum immature and cardiac calcium stores reduced
- ventricles less compliant: CO is HR dependent
- baroreceptor reflex immature, won’t have increase in HR in response to decrease BP
- Heart not as responsive to volume c/t adult
- PSNS dominance- immature SNS, and much more likely to have bradycardia with any kind of stress/suctioning/ etc
- premedicate prior to DL/suction etc
Resting cardiac ouput for neonate, infant, and adolescent?
- Neonate at birht 400mL/kg/min
- Infant 200 mg/kg/min
- Adolescent 100 mL/kg/min
CV characteristics in neonate?
- Dependence on ionized calcium- particularly vulnerable to effects of citrated blood products
- also vulnerable to myocardial depression caused by potent anesthetics
- Neonate myocardium relatively noncompliant c/t older kids
- increased preload does not increase SV to same degree
- poor tolerance to increase afterload (development of BiV failure)
- hypovolemia and bradycardia produce dramatic decrease in CO that threaten organ perfusion
- Epinephrine rather than atropine increases contractility and HR
- preferred txmt of bradycardia and decreased CO in ped patients
- In 1st 3 months, heart does not respond as well to inotropic support
- immature beta respons
*
- immature beta respons
Pulmonary system in infants?
- Alveoli increase in number & size up until 8yo
- Infants: small airway diameter; increased resistance
-
Highly compliant airway & chest wall
- however, lung tissue not as compliant. less elastin tissue. this can lead to airway collapse and chest wall collapse
- Closing capacity is greater than FRC in the very young and very old: airway closure can occur before end exhalation
- Early fatigue of diaphragmatic & intercostal muscles until age 2 (type 1 muscle fibers not mature)
- only 10% type 1 muscle fibers in the diaphragm in infant. adult has 55%
-
Highly compliant airway & chest wall
-
O2 consumption is 2-3 x’s the adult with increased alveolar ventilation; leads to rapid desaturations especially during cold stress and in the case of airway obstruction
- MV: FRC ratio 2-3 x higher than adult causes faster anesthetic onset, fast desat and less O2 reserve
- Angulation of right mainstem bronchus

Airway differences in infant?
- Infant:
- larger tongue in smaller submental space
- higher larynx(C2 to C4)
- short stubby (omega shaped) epiglottis
- angled vocal cords (slant caudally)<bolded></bolded>
- funnel shaped larynx with narrowest region @ cricoid ring
- obligate nasal breathers
- large occiputs & the “sniffing” position is favored for axis alignment
- shoulder roll useful. large head c/t body, no hyperextension!
- endentulous
- short trachea (4-5 cm)
- Tooth eruption normally occurs between 4 and 12 months of age for the first tooth; eruption of the 20 primary teeth should be complete between 24 and 30 months of age.

Gas flow in young children?
- Young children have elevated airway resistance at baseline
- Turbulent airflow is present to 5th bronchial division
- A 50% reduction in radius increases the pressure 32-fold
- Very prone to respiratory distress with any upper airway irritation or swelling
- laminar flow R to 4th power (poiseuille’s law!)
- turbulent radius to 5th power–> even more reduction in flow

Neurological characteristics of infant?
O2 consumption? Growth of brain? Location of conus medullaris?
Fontanel closure (ant and post)?
- Oxygen consumption & CBF in the brain of children is ~50% greater than adults
- O2 consumption
- infant 5.5 mL/100g/min
- adult 3.5 mL/100g/min
-
CBF
- infant 100 mL/min/100g
- adult 50 mL/min/100g
- O2 consumption
- Myelinization & synaptic connections not complete until age 3-4 years
- Rapid growth of brain in first 2 years of life
- Conus medullaris is at level of L3 at birth & migrates to level L1-L2 by age 3
- Fontanels: anterior fontanel closed by 18 mo’s; posterior fontanel closed by ~2 mo’s
What is anesthesia induced developmental neurotoxicity?
Anesthesia-Induced Developmental Neurotoxicity: our knowledge is still growing in this area
- Increased and accelerated neuroapoptosis with virtually all anesthetics
- Single exposures of short duration are usually of no consequence
-
Repeated &/or prolonged exposures at a young age (<3-4 years) may be associated with later behavioral & learning difficulties- we do not have conclusive evidence
- __current thought to delay elective/non-urgent sx until children >3-4 yrs from neurocognitive standpoint. have not proven delays
-
most GA cause morphology changes in developing brain
-
some human sutdies have gound association b/w exposure to aneshesia and surgery in early childhood
- may be explained by confounding factors
- increasing evidence shows one hour of aneshteisa in infancy does not have lasting impact on cognition
-
some human sutdies have gound association b/w exposure to aneshesia and surgery in early childhood
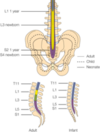
Neuraxial considerations in pediatrics
- The conus medullaris ends at approximately L1 in adults and at the L2–L3 level in neonates and infants.
- In infants, the line across the top of both iliac crests (the intercristal line) crosses the vertebral column at the L4–L5 or L5–S1 interspace, well below the termination of the spinal cord
- The dural sac in neonates and infants also terminates in a more caudad location compared to adults, usually at about the level of S3 compared to the adult level of S1
- Infants: lack of a lumbar lordosis compared to older children predisposes the infant to high spinal blockade with changes in positioning
Renal characteristics of infants?
-
GFR is significantly impaired at birth but improves throughout the 1st year
- greatest impairment is in 1st 4 weeks of life
- renal maturation will be delayed further with prematurity
- UOP low at birth x 24 hours then increases to 1-2mL/kg/hr
- be concerned after 24 hours with low UOP
- in utero kidney only receives 3% blood flow. adult 25%.
- Renal tubular concentrating abilities do not achieve full capacity until ~2years
- difficulty with concentrating and diluting urine
-
does not respond as well to aldosterone
- hypo/hypernatremia can easily become an issue
- Half-life of medications excreted by glomerular filtration are prolonged in the very young (antibiotics; etc.)
- In contrast, during childhood, renal clearance rate may increase to levels higher than even adult clearance rates
- higher CO, more blood flow in childhood
Liver function in infants?
- Enzyme systems are still developing up until 1 year of age
- Phase I Cytochrome P450 system is 50% of adult values at birth
- 3A4 50% drugs
- 2D6= 10-20% drugs
- Phase II (conjugation reactions) are impaired in neonates
- Long half life of BZD and morphine
- Decreased bilirubin breakdown due to reduction in glucuronyl tranferase (leading to jaundice)- also metabolize tylenol
- Hepatic synthesis of clotting factors reach adult levels within a week of birth
-
Vit K dependent factors (II, VII, IX, X)
- at birth 20-60% adult values
- preterm values even less
-
Vit K dependent factors (II, VII, IX, X)
-
Lower levels of albumin/ other proteins for drug binding in newborns- larger proportion of unbound drug circulating
- increases effect of highly protein bound drugs.
- Minimal glycogen stores- prone to hypoglycemia
GI system in pediatrics?
- Obligate nose breathers
- Coordination of swallowing with respiration not mature until 4-5 months of age (grow out of it eventually)
- high incidence of reflux especially in pre-terms
- coanal atresia- blockage of nasal to trachea
- resp depression bc want to breathe through nose! Will breath better when crying
- coanal atresia- blockage of nasal to trachea
-
Gastric juices are less acidic (more neutral) up to ~3 years of age
- Less absorption of drugs
-
Absorption of oral medications is generally slower compared to adults (less effective)
- The gastrointestinal tract is generally slower in children than in adults
- Children have differences in gastric pH, emptying time, intestinal transit, immaturity of secretions, and activity of both bile and pancreatic fluids
What are factors that lead to difficulty in thermoregulation in infants?
- Large surface area to body weight
- Lack of subcutaneous tissue as an insulator
-
< 3 mo →Inability to shiver:metabolize brown fat to increase heat production
- can lead to metabolic acidosis & increased O2 consumption
- Brown fat: tissues in neck, vertebral column, around adrenal glands → Metabolically stressful!
- can lead to metabolic acidosis & increased O2 consumption
-
Factors: cold OR, anesthetic-induced vasodilation, room-temp IV fluids, evaporative heat loss from surgical site, cool irrigating solutions on field, cool/dry anesthetic gases
- In picture
-
Conduction- cold fluid, cold OR table (cold surface/fluid absorbing heat)**
- decrease heat loss by placing baby on warming mattress and warm surgical unit
-
Evaporation- cold gas vent to pt, cold irrigating fluid r/t heat loss
- humidifaciton of inspired gases, use plastic wrap and warm skin disinfectant solutions
-
Convection- air flowing over
- keep infant in incubator and cover with blankets
- head should be covered
-
radiation from image- giving off heat
- use double shelled isolette during transport
- % of heat loss in children: 39% radiation; 34% convection; 24% evaporation; 3% conduction

How can we maintain temperature in infants?
-
Active warming is critical:
- warm the OR (dec convection) 72-76o (or 80’s)
- as warm as everyone can tolerate in infants
- use a warming mattress
- use incubators
- cover with blankets- dec radiation
- head coverings (up to 60% of heat loss)
- transport in isolette
- humidify gases- dec evaporation
- single limb circuit**- gases getting warmed up by exhaled air
- use plastic wrap on the skin
- warm prep & irrigation solutions
- change wet diapers & remove wet clothing
- Forced air warmers: the most effective strategy to minimize heat loss in surgery in children > 1 hr
- Careful w/ injury!
- warm the OR (dec convection) 72-76o (or 80’s)
- Anesthetics alter non-shivering thermogenesis in neonates
What are some best practices for temperature monitoring in pediatrics?
- Essential for all pediatric cases
-
Mid-esophageal placed probe- best core temp!
- can also be used as stethoscope
- Axillary temp: Advantage - if properly positioned:
- proximity to deltopectoral group improves recognition of elevated temp in MH
- NO FOREHEAD TEMP- not advised
- 10 MH episodes occurred that were unrecognized with forehead temp (Barash)
-
Hypothermia: consequences →
- delayed emergence- metabolism of drugs slower
- reduced degradation of drugs
- increased infection
-
Hyperthermia: MH? → primary presentation not always fever (1st see ETCO2)
- Stop VA, high flow on, switch to TIVA, evaluate (stop anesthetic d/t Ca dysregulation getting worse)
Body composition of infant? Electrolytes?
-
Total body water is highest in premature infants & decreases with age
- Larger SA per Kg
- Metabolism correlates to O2 consumption, CO2 production CO, alveolar vent
- Better to look at Body SA (BSA) rather than wt (better measure of metabolism)
- Infants: ECF > ICF
- Adults: ECF < ICF
- Infants: don’t have ICF reserve → cant pull from when dehydrated (??)
- Childhood: ICF proportion increases → reserve for dehydration
- Electrolytes:
- Same as adult → but know:
- Issues w/ Na levels
- K slightly higher 1st 1-2 days
- Same as adult → but know:

How can body composition of infants alter pharmacokinetics?
- Water soluble drugs have a larger volume of distribution (have higher TBW)
- Need a larger initial dose (Sch; abx- higher dosing of water soluble drugs)
- Delay excretion – from larger volume of distribution
-
Acidic drugs tend to bind mainly to albumin (e.g., diazepam, barbiturates)
- plasma protein binding of many drugs is decreased in the neonate relative to the adult in part because of reduced total protein and albumin concentrations.
- Basic drugs bind to globulins, lipoproteins, and glycoproteins. (e.g., amide local anesthetic agents)
-
Half-life of medications in >2 years of age is shorter than adults or equivalent due to significant CO to liver & kidneys
- More fentanyl/propofol mg/kg
- Pharmacokinetics in children varies with body composition, renal and hepatic function, and with altered protein binding
- Neonates have less fat & muscle
- Drugs that depend on redistribution to fat for termination of action will have prolonged effects (fentanyl; propofol)
- Protein binding: < 6 months old have reduced albumin & alpha-1 acid glycoprotein (AAG)
- higher free-fraction of protein bound drugs → higher risk of toxicity!!
- Free fraction of lidocaine will be higher in the very young!
- higher free-fraction of protein bound drugs → higher risk of toxicity!!
“In general, most medications will have a prolonged elimination half-life in preterm and term infants, a shortened half-life in children older than 2 years of age up to the early teenage years, and a lengthening of half-life in those approaching adulthood.” – patient to patient variability ***
Difference in drug pharmacokinetics in infant, childhood to adulthood?
- Preterm/infants: prolonged elimination half-life
- >2 yo to early teenage yrs: shorted half-life
- Adulthood: lengthened half-life
Difference in hematocrit and blood volume in infant?
How do we dose blood transfusions in infants?
Fetal Hgb:
- Lower P50 (19 mmHg vs. adult normal of 26 mmHg) → left shift = Holds onto O2!
- Low levels of 2,3 diphosphoglycerate
- This lower P 50 allows the fetus to load more oxygen at low placental oxygen tension, but it makes unloading oxygen in tissues more difficult.
-
3- 6 months after birth → fetal hemoglobin has been replaced with adult hemoglobin.
- Tolerate Anemia more poorly bc left shift
- Blood products helpful d/t having adult Hgb that allows released O2 to tissues
-
Target hct in neonates is higher
- Hct minimum 40% (instead of 30%)
- Why?: bc
- L shift
-
Tx: 4-5 ml/kg of transfused PRBC’s increase hgb ~1g/dL
- Order blood based on body weight
- Ex: 15 kg pt = 75 ml blood
What is physiologic natar?
- Physiologic Natar: lowest point of anemia as fetal Hgb being replaced (PERIOD OF TRANSITION)
- Physiologic anemia at 2-3 months of age→ lower threshold to give blood products (low P50 & physiologic anemia)
How do you calculate MABL?
Considerations for blood transfusions threshold?
-
Maximum allowable blood loss calculation:
-
MABL= EBV x (starting HCT- target HCT)/ starting HCT
- variables: EBV, patient starting hct (est by chart w/o labs), minimum allowable hct
- *gives threshold when need to start giving blood
- Threshold varies (ex: ~30%)
- Ex: < 3 mo → higher threshold d/t physiologic anemia & low P50
- variables: EBV, patient starting hct (est by chart w/o labs), minimum allowable hct
-
MABL= EBV x (starting HCT- target HCT)/ starting HCT
- Initial blood loss replaced at 3:1 with crystalloid
- Transfusion threshold: somewhere around hct ~24- 30%; minimal target hct should be discussed based on individual child
- consider blood sooner in the following: (higher tx threshold)
- preterm infants
- term newborns
- children with cyanotic congenital heart disease
- children with respiratory failure in need of high oxygen-carrying capacity
- young infants (< 3 months) may need a higher transfusion threshold due to left shift & physiologic anemia
- incidence of apnea is high in neonates and preterm infants who have hematocrit values < 30%
- consider blood sooner in the following: (higher tx threshold)

How do you determine how much blood to transfuse in infant?
- Once MABL is approaching, if blood loss is expected to continue then blood will be given
- always use a blood warmer
- Calculation of blood to be transfused: (desired hct - current hct) x EBV
hct of PRBC’s (which is 60%)
- > 1 blood volume replaced → FFP will be needed
- Watch for ionized hypocalcemia & resultant CV depression (esp w/ rapid infusion of FFP)
- Reasons for it being risk:
- Ca stores already low in neonates
- FFP has highest concentration of citrate
- neonates & children with liver failure are at pronounced risk
- Reasons for it being risk:
-
Platelets: need for replacement depends on starting platelet count- clinical oozing on the field is the typical indicator
- Starting normal platelet count usually does not need platelets UNTIL EBL > 2 blood volumes

Average Hgb/HCT for newborn, 3 month, 6-12 months and adult female/male?
EBV for preterm neonate? term neonate? infant? >1 yr?

Coagulation in newborns/infants?
- At birth, vitamin K-dependent coag factors are low ( 2,7, 9,10)
- reach adult levles by 6 months age
-
fibrinogen polymerization does not reach its full capacity during first few postnatal months
- leads to prolonged thrombin time
-
PLT number at birth comparable to adults
- however, PLT function impaired in early life
-
Postnatal period represents hypercoaguable state
- d/t inhibitor of coagulation decreased by 30% to 50% in newborn
- Antithrombin III and protein S levels reach maturity by 3 months of age
- protein C and plasminogen levels reach adult levels after 6 months of life
- higher risk for thrombotic complications in neonates and infants.
Standard components of fluid replacement in pediatrics?
- Components of fluid replacement:
- Fasting (NPO) deficit (maintenance rate x hours NPO for deficit)
* don’t always replace NPO def in healthy child for elective procedure
- Fasting (NPO) deficit (maintenance rate x hours NPO for deficit)
- Baseline maintenance fluid requirement- using LR (balanced salt solution- not dextrose unless risk pop) in most cases (4:2:1)
- Replacement of blood loss- hourly
* (3:1 crystalloid replacement until MABL reached then 1:1 colloid (blood)
- Replacement of blood loss- hourly
- Evaporative losses (based on invasiveness of surgery)
- Now believe holliday segar not necessary
- Miller refers to using holiday segar for infnats <6 months. >6months 10-40mL/kg over 1-4 hours appropraite for NPO and replacement.

Fluid replacement considerations in pediatrics?
Dehydration guidelines?
- LR is typically used for maintenance in healthy children
- < 6 mo’s & in others at risk for hypoglycemia: glucose containing IVF may be needed in infants
- Ex: D51/2
- < 6 mo’s & in others at risk for hypoglycemia: glucose containing IVF may be needed in infants
- Minimize potential for error: smaller IV bags (250/500 ml bag); buretrols
-
Eliminate all air from IV line
- Volume of air and rate of entrainment leads to VAE
- If PFO opens because of surgical stress, air will go straight up to brain
- Recognize dehydration in infants: the best measure of deficit is weight
- Mild: ~50ml/kg deficit dry mouth, poor skin turgor
- Moderate: ~100ml/kg mild sx plus deficit sunken fontanel, oliguria, tachycardia
-
Severe: ~150 ml/kg moderate sx plus sunken eyes, hypotension, & anuria
- Pulse pressure really useful, respiration,
Considerations of glucose and hypoglycemia prevention in infants?
- Routine use of glucose-containing IVF in the perioperative setting in children is NOT recommended
- Exception: Children at high risk of hypoglycemia- can use D5 1/2NS @ maintenance rates
- Don’t use for BL or evaporative loss replacement (must use balanced Na solution)
-
Continuous TPN:
- must NOT suddenly stop
- consider leaving on at a reduced rate
- some providers may use D10 to bridge- monitor glucose!!
- Children with mitochondrial disease will definitely need glucose containing replacement fluid
- Exception: Children at high risk of hypoglycemia- can use D5 1/2NS @ maintenance rates
Miller says cut the TPN rate by one third and leave running
Why is uptake more rapid in children?
Uptake (Wash-in) more rapid in children for several reasons:
- increased respiratory rate
- increased cardiac index<< this is somewhat contradicted in Miller? Miller says uptake is dependent on CO. We learned before increase CO= slower onset?
- larger proportion of blood to VRG (heart, brain, GI, kidneys, endocrine)
- Reduced tissue/blood and blood/gas solubility in infants
- Increased Alveolar ventilation to FRC ratio
- Infants: 5:1
- Adults: 1.5:1
-
*Increased risk of anesthetic overdose in infants/ toddlers
- Faster equilibration to what set on dial (from co-exist lecture last semester)
-
Determinants of “wash in” of VA → FRC, inspired concentration, alveolar ventilation
- Wash in is inversely related to solubility= lower solubility→ higher wash in
- Less is binding to tissue, less dissolved in blood
- Wash in is inversely related to solubility= lower solubility→ higher wash in
- Removal → CO, solubility, alveolar-venous partial pressure
-
Determinants of “wash in” of VA → FRC, inspired concentration, alveolar ventilation
- Faster equilibration to what set on dial (from co-exist lecture last semester)
- 18% BF to VRG in infants as opposed to only 8% in adults
MAC for sevo and des in neonate, infant and children?
- Sevoflurane:
- Neonates: 3.3%
- Infants (1-6 mo): 3.2%
- Children (> 6 mo): 2.5%
- Desflurane:
- Neonates: 9.2%
- Infants (1-6 mo): 9.4
- Infants (6-12 mo): 9.9%
- 1-3 yo: 8.7%
- 5-12 yo: 8%
- All VA: MAC increases until 2 to 3 months of age (max: 1 to 2 years old) and steadily declines with age thereafter
- Sevo (Exception): MAC remains constant in neonates and infants up to 6 months
- MAC up to 6 months is ~3.2%
- MAC 6 months to 12 years is constant at 2.4% (decrease)

Can you use a BIS monitor in kids?
No, children have higher BIS for a specific fraction of MAC c/t adults
Use of various VA in neonates and infants?
- Increase hypotension incidence in neonates & infants upon inhalational induction
- More rapid uptake can unmask negative inotropic effects of the volatiles in infants
Agents and use:
-
Sevoflurane – primary agent for inhalational induction
- Well tolerated by mask
- No irritation of airway (least pungency)
- Potent bronchodilator
- Halothane
- low pungency
- no longer used in US
- bradycardia at induction (higher solubility- slower onset, phase 2, laryngospasms)
- most dangerous → MAC multiples if turned all the way up, go up to 5.7x MAC (risk of overdose)
- Desflurane – limited use
- Pungent!
- Leads to: Breath holding, coughing, salivation, laryngospasm
- Low solubility → can use for maintenance for long sx/obese children
- Faster wake up
- Pungent!
Respiratory and CV effects of various inhaled anesthetics in pediatrics?
- Respiratory: same as adults:
- Dose related respiratory depression
- decrease response to CO2 & hypoxia
- As concentration increases, apnea ensues
- CV:
-
dose dependent depression
- Myocardial depression may be exaggerated in neonates d/t:
- immaturity of sarcoplasmic reticulum → more susceptible to calcium-channel blocking effects of volatile anesthetics
- Delicate balance of achieving adequate depth at induction & avoiding cardiovascular depression
- Myocardial depression may be exaggerated in neonates d/t:
-
dose dependent depression
- all can cause prolonged QT
- Sevo usually maintains or increases HR during induction
-
halothane has greatest depression of contractility → induction arrests! Over pressuring during induction and had negative inotropic affect
- Halothane: high lipid solubility; slow onset & offset; low pungency; no longer used in US
- Halothane hepatitis- antibody reaction; repeated exposure
- Isoflurane:
- limited utility for induction due to noxious smell
- increased risk of laryngospasm/ coughing
- confers more cardiac stability (as in adults)
- Emergence delirium is commonly associated with all inhaled agents (sevo>des>halothane)
- Halothane less likely bc comes off slower
Induction agent use in pediatrics?
Propoofl, ketamine, etomidate, thiopental, methohexital doses?
-
Neonates: Immature BBB & decreased metabolism can increase sensitivity
- increased permeability of BBB makes more sensitive
Older children & adolescents generally require increased doses of induction agents compared to adults
- Dosing:
-
Propofol (Diprivan): have extra available
- < 2 yo: 2.9 mg/kg
- 6-12 yo: 2.2 mg/kg
-
Ketamine:
- 2 mg/kg IV
- 4-8 mg/kg IM (plus atropine 0.02 mg/kg IM/IV → for hypersalivation)
-
Etomidate:
- 0.25-0.3 mg/kg
-
Thiopental sodium (Pentothal):
- neonates (< 1 month): 3 to 4 mg/kg
- infants (1 m–1 yr): 7 to 8 mg/kg
- Children: 5-6 mg/kg
-
Methohexital: ECT therapy
- 2 mg/kg IV or 15-25 mg/kg of a 1% or 20-30 mg/kg of a 10% solution PR
-
Propofol (Diprivan): have extra available
Propofol use in pediatrics?
- Most commonly used IV induction agent in children
- Greater Vd than adults
- More rapid redistribution
- Pain of injection can be reduced with a mini Bier block with 0.5-1 mg/kg of Lidocaine for 60 seconds (BP cuff)
- Antiemetic properties
- TIVA- lower rate of PONV/emergence delirium
- Propofol infusion syndrome: long term infusions in ICU avoided in infants & children (acidosis); still appropriate for TIVA case
- Egg/soy: only avoid if documented anaphylaxis with eggs
Ketamine use in pediatrics?
- can be used IM, IN, PO, IV → hemodynamic compromised pts
- Induction with ketamine preferred in
- severe hypovolemia,
- cyanotic heart disease,
- septic shock, & induction for mediastinal mass (need spontaneous ventilation)
- Increased secretions (premedicate w/ anticholinergic)
- Ex: atropine
- Emergence irritation
- reduced with co-administration w/ Midazolam
- waking up in a dark/quiet room
- Induction with ketamine preferred in
Etomidate use in children?
- only approved for use in age >10 yo in U.S (0.2-0.3 mg/kg IV)
- Pain on injection
- Adrenal suppression concern
Midazolam use as sedatives in pediatrics?
Metabolism?
Reversal agent?
most widely used anxiolytic pre-op
- Oral dosing:
- dose increases in younger patients
- poor oral bioavailability
- bitter taste
-
allow 10-15 minutes
- ORAL dosing: dose decreases w/ age
- 18 mo-3yo: 0.75-1 mg/kg
- 3-6 yo: 0.6-0.75 mg/kg
- 6-10 yo: 0.5 mg/kg 6-10 yo
- ORAL dosing: dose decreases w/ age
- IV: 0.1-0.2 mg/kg (immediate onset)
- Intranasal: 0.3 mg/kg
- MAX DOSING: 15 mg
- Reversal:
- flumazenil 0.01mg/kg IV
- Hepatic metabolism (CYP 3A4) & renal excretion
Ketamine use in peds as sedatives?
- Severe cognitive/ behaviorally challenged older children → IM administered preop ** preferred
- IM dose: 4-5 mg/kg
- Onset: 3-5 minutes
- Duration: 30-40 minute duration
- Oral onset: 15-20 min
Dexmedetomidine as sedative in pediatric patients?
- hypotension with loading doses
- bradycardia with high dose infusion
- (note biphasic response with transient hypertension initially) → then hypotension
- will not be adequate as a sole anesthetic but can be helpful as adjunct
- opioid sparing effects
- Uses:
- useful in awake FOB
- radiological procedures
- reduction of emergence delirium
- Uses:
- Dosing:
- IV:
- initial dose: 0.7-1.0 μg/kg administered over 10 minutes
- → followed by infusion: 0.5-1 μg/kg/hr
- Intranasal: 80% bioavailability!! great premed!! But takes long to peak
- Dose: 1-2 mcg/kg
- Peak: 30-40 min (long time)
- IV:
Considerations of opioids in pediatrics?
morphine? fentanyl? dilaudid? remi? demerol? codeine?
-
Variety of choices: onset, potency, duration, & metabolism are factors just like in adults
- Also consider previous exposure to opioids (tolerance), severity of pain, & other multi-modal strategies
-
Morphine:
- active metabolite (morphine-3- glucuronide)
- → cause prolonged respiratory depressant effects in neonates and preterm and critically ill infants
- Dosing: 50-100 mcg/kg (IV) per single dose
- active metabolite (morphine-3- glucuronide)
- Dilaudid dosing: 10-20 mcg/kg (IV) per single dose
-
Fentanyl: most widely used opioid intra-op in children
- stable CV profile
- Caution: chest wall rigidity
- Dosing variability
- ~ 0.5-1 mcg/kg range
- Work up to 1-3 mcg/kg range (IV) per single dose & titrated for effect
- Safely go up to 10 mcg/kg depending on sx
- ~ 0.5-1 mcg/kg range
-
Remifentanil:
- excellent for neonates
- great choice in renal/hepatic failure/immaturity (due to metabolism via esterases → mature system in neonate)
- predictable eliminiation
- Caution:
- bolus injections can cause significant bradycardia/hypotension
- must have plan for analgesia once infusion discontinued
- Dose: 0.05-0.1 mcg/kg/min
- excellent for neonates
-
Demerol:
- Admin for shivering in small doses (0.25-0.5 mg/kg)
- Metabolite: normeperidine
-
Codeine:
- historically very commonly prescribed postop
- withdrawn from many markets due to respiratory events
- SNP’s in ultra-rapid metabolizers confer risk of OD (CYP affected)→ BLACK BOX warning
- Slow metabolizers- placebo
Acetaminophen and ketorolac use in peds?
- Acetaminophen:
- PO 10-15 mg/kg
- IV: 15 mg/kg q 6 hours (10-15 min onset)
- rectal absorption: SLOW (1-2 hours)
- Ketorolac:
- Dose: 0.5 mg/kg IV
- Ask surgeon before administration
- CAUTION:
- Severe asthma → caution all NSAIDs
- Nasal polyps, eczema, and asthma → tend to have NSAID allergies
- Severe asthma → caution all NSAIDs