Pathophysiology and Treatment of Type I Diabetes Mellitus Flashcards
Name a form of type 1 diabetes that presents late.
Latent Autoimmune Diabetes in Adults (LADA)
NOTE: Form of type 1 diabetes because it is autoimmune
State some features of T2DM which could cause ambiguity when trying to identify the type of diabetes someone is affected with.
T2DM:
- May present is childhood
- Has diabetic ketoacidosis as a feature
These could cause ambiguity as they are commonly considered features of T1DM
State two monogenic causes of diabetes.
Maturity Onset Diabetes of the Young (MODY)
Mitochondial diabetes
NOTES:
- Monogenic = single gene defect
- MODY
- Various single gene defects can be responsible
- Mitochondrial diabetes
- Single gene defect in mDNA impacts ATP production
- In beta cells ATP required for insulin release (ATP sensitive K+ channels)
- Therefore if ATP production is disrupted, then insulin release is disrupted
Following what may diabetes present?
Diabetes may present following:
- Pancreatic damage
- Other endocrine disease
Describe the relative presence of different types of diabetes.
The bigger the circle, the more prevalent

Describe the current classification of type 1 and 2 diabetes mellitus.
Based on aetiology (cause)

Which type of diabetes has a cause with a bigger genetic infuence?
Type 2 Diabetes Mellitus
What can be measured in the blood to give an indication of insulin production?
C-peptide
- Part of proinsulin molecule - cleaved in the insulin synthesis process
- Once cleaved, the C-peptide and mature insulin are both stored in the secretory granules of the beta cells so both are released at the same time
- C-peptide has a longer half life than insulin
Describe the pathogenesis of T1DM.
- You get gradual autoimmune destruction of beta cells resulting in gradually reducing levels of insulin (and C-peptide)
- i.e. Antibodies directed against beta cells
- You get a prediabetic phase of elevated plasma glucose but some insulin is still being produced
- One of the first signs will be the loss of first phase insulin response (FIPR)
- FIPR is release of stored insulin when glucose is given IV - very fast
- Loss of this is one of the first signs of impaired insulin secretion
- There will be eventual destruction of all beta cells → severe insulin deficiency
- Overt = easily observable phase

Why is T1DM described as a ‘relapsing-remitting’ disease?
- Over time the beta cell mass appears to reduce, then stabilise, then reduce again
- There is a theory that this is due to the imbalance in effector T-cells and regulatory T-cells
- Effector T cells cause the destruction of beta cells
- Regulatory T cells control this destruction
- Initially an increase in the numbers of autoreactive effector T cells is controlled by an increase in the number of regulatory T cells - cyclical pattern
- However, over time, a gradual disequilibrium of the cyclical behavior could occur, leading to the number of autoreactive effector T cells surpassing the number of regulatory T cells
- This leads to autoimmune destruction of beta cells to the point where you no longer have enough to produce sufficient insulin to control blood glucose levels anymore
- The stabilisation may also be due to increase in beta cell proliferation over time which could be triggered by the inflamatory process
NOTE: Honeymood phase = the period of time shortly following diabetes diagnosis when the pancreas is still able to produce a significant enough amount of insulin to reduce insulin needs and aid blood glucose control
- BUT once these cells die you will have hyperglycaemia

What is the importance of the autoimmune basis of T1DM?
- Increased prevalence of other autoimmune disease
- Risk of autoimmunity in relatives
- More complete destruction of B-cells
- Autoantibodies can be useful clinically
- As a marker of certain diseases
- Immune modulation offers the possibility of novel treatments
What are the histological features of T1DM?
Lymphocyte infiltration of beta cells
- Islets of Langerhans (shown in the picture) are mainly made up of beta cells
- Lymphocytes lead to production of auto-antibodies and beta cell destruction

On which chromosome is the HLA found?
Chromosome 6
NOTE:
- HLA = human leukocyte antigen
- In humans the MHC proteins are coded for by the HLA gene complex/group
Which alleles convey a risk of diabetes? Which of these alleles is associated with the most significant risk?
DR alleles
- DR3 and DR4 = significant risk
NOTE: HLA-DR is one of the isotypes of MHC II proteins

Describe how environmental factors may affect type 1 diabetes.
T1DM prevalence:
- Increased in autumn
- Decreased in summer
Theory:
- There may be certain pathogens in the environment that trigger the onset of diabetes but is more prevalent in certain seasons
- Infections that target beta cells and promote strong inflammation within the islets may induce autoimmunity
State some antibody markers of type 1 diabetes.
- Islet cell antibodies (ICA) - blood group O human pancreas
- Insulin antibodies (IAA)
- Glutamic acid decarboxylase antibodies (GADA)
- GAD synthesises GABA which is a widespread nuerotransmitter
-
GABA is released by beta cells to have a paracrine function
- e.g. activation of GABA receptors in beta cells increases insulin release
- Insulinoma-associated-2 autoantibodies (IA-2A)-receptor like family
- Insulinoma-associated protein 2 is associated with the membrane of secretory granules
- If these are affected it would lead to reduced insulin release
State some symptoms of T1DM.
Symptom = experienced by the person who has the condition
- Polyuria
- Nocturia
- Polydipsia
- Blurring of vision
- Short term - hyperglycaemia can lead to swelling of the lens, which can result in temporary blurring of eyesight
- Long term - diabetic retinopathy
- Thrush
- Poorly controlled diabetes leads to increased risk of infection as the hyperglycaemic enviroment leads to immune system dysfunction
- Weight loss
- Fatigue
- Insulin facilitates glucose uptake into cells so lack of insulin results in reduced uptake (e.g. muscle cells)
- Reduced uptake means reduced glucose avalailable for ATP production
What are the signs of T1DM?
Sign = the effect of a health problem that can be observed by someone else
- Dehydration
- Cachexia
- Cachexia = severe weight loss and muscle wasting
- Leads to extreme weakness
- Hyperventilation (Kussmaul breathing)
- Kussmaul breathing = deep laboured breathing that occurs in response to severe metabolic acidosis (in this case: diabetes ketoacidosis)
- Smell of ketones
- Glycosuria
- Ketonuria
What are the triglycerides in adipocytes broken down to?
Glycerol
Fatty Acids
What does insulin have an inhibitory effect on?
- Hepatic glucose output (i.e. glucose release from liver)
- Protein breakdown in muscle
- Glycerol release from the adipocytes
- Ketone body generation by the liver
What does insulin have a stimulatory effect on?
Glucose uptake by muscle
Describe some of the processes which take place if you are insulin deficient.
Hyperglycaemia
- You lose the inhibitory effect on insulin on hepatic glucose output (HGO) so you get more glucose being released into the bloodstream from the liver
- You lose the stimulatory effect of insulin on glucose uptake, so you get reduced glucose uptake into muscle
- This exacerbates the hyperglycaemia
Other metabolic effects
- You get increased breakdown of muscle protein as this is no longer being inhibited by insulin
- The AAs are taken up by the liver and the glucogenic ones can be used in gluconeogenesis
- You get increased release of glycerol from adipocytes which is taken up by the liver and used in gluconeogenesis
- Gluconeogenesis → increased HGO

State the hormones which oppose insulin action.
Increase HGO:
- Catecholamines
- Cortisol
- Growth hormone
- Glucagon
Promotes muscle protein breakdown:
- Cortisol
Promote glycerol release from adipocytes:
- Catecholamines
- Growth hormone
Promotes ketone body formation in liver:
- Glucagon
Describe how insulin deficiency leads to diabetic ketoacidosis (DKA).
Insulin has an inhibitory effect on:
- Glycerol AND fatty acid release from the adipocytes
- Essentially insulin inhibits lipolysis
- Ketone body generation by the liver
Process:
- Triglyceride breakdown → glycerol and fatty acids
- Released into circulation due to lack of inhibition by insulin
- Fatty acids enter liver and are converted into ketone bodies as it is not being inhibited by insulin
- These ketone bodies enter:
- Blood → diabetic ketoacidosis as they are acidic
- Urine → ketonuria
- Muscle → provides energy
- Some ketones are taken up by muscle but it is not as good of a fuel as glucose

What is the main treatment for T1DM? What are the main aims of treatment?
Exogenous insulin
Main aims:
- Reduce early mortality
- Prevent long-term complications
- Avoid acute metabolic decompensation
- i.e. The functional deterioration of a system that had been previously working with the help of compensation
-
Deterioration - i.e. loss of glucose homeostasis
- hyperglycaemic state
- diabetic ketoacidosis
What is a defining feature of insulin deficiency?
Ketone bodies
NOTE: some cases of T2DM can also get DKA but this is mainly a complication of T1DM
State some long-term complications of T1DM.
- Neuropathy
- Nephropathy
- Retinopathy
- Vascular disease
Describe the dietary changes that are recommended in T1DM.
- Reduce calories as fat
- Reduce calories as refined carbohydrate
- i.e. carbohydrate-rich foods which have been processed and have had other important nutrients and fibre
- Increase calories as complex carbohydrate
- This essentially refers to polysaccharides - i.e. starch
-
They are digested more slowly and supply a more steady release of glucose into the blood stream
- i.e. don’t increase plasma glucose levels quickly
- Increase soluble fibre
- Can slow the absorption of glucose from the small intestine
- Balanced distribution of food over course of day with regular meals and snacks
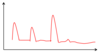
Describe the insulin profile of a non-diabetic.
3 main peaks - one after each meal
Basal insulin:
- Low (basal) levels of insulin being produced by the pancreas througout
-
This allows blood glucose levels to be regulated while fasting
- Keeps blood glucose levels under control when glucose is being released by energy stores
- Allows this glucose to be taken up by other body tissues to provide them with energy
Describe insulin treatment.
With meals (i.e. bolus insulin):
- Short acting
- Human insulin
- Insulin analogue (Lispro, Aspart, Glulisine)
In the background (i.e. basal insulin):
- Long acting
- Non-C bound to zinc or protamine
- Gives the insulin longer half-life
- They are absorbed slowly, then reach a consistent level (no peak effect) and have a long duration of action
- Non-C probably means without the C-peptide
- Insulin analogue (Glargine, Determir, Degludec)
What is the basis of insulin treatment?
Genetic engineering of different types of insulin to alter absorption, distribution, metabolism and excretion
- This allows treatment to mimic normal physiology
Describe what treatment you would give a patient who has meals twice a day and what the insulin profile would look like.
NOTES:
Intermediate-acting insulin:
- Intermediate duration of action and half-life
- Takes longer to have an effect than short-acting insulin
- Has a peak but it is not as high as short-acting insulin
- Given as a supplement to basal insulin
Arrows represent meals

Describe 2 the two different treatments you could give in patients who have 3 meals a day (i.e. most patients) and what the insulin profile would look like.

What do insulin pumps do?
- Continuous insulin delivery
- There are pre-programmed basal rates and bolus for meals
- But these DO NOT measure blood glucose so the feedback loop isn’t complete
- It can’t detect blood glucose and alter insulin deliver based on that
- Therefore it cannot completely replace beta cell function
Describe the use of islet cell transplants.
Procedure:
- Islet cells extracted from healthy pancreas
- Inserted into patient’s liver through portal vein
- They can distribute themselves around the body
Main problem:
- Patient must be on immunosuppressants for life
How is capillary monitoring done and what does it give a measure of?
Procedure:
- Prick the finger and test the glucose levels in the blood drawn
Advantage:
- Gives you a trend of blood glucose levels throughout the day
Disadvantage:
- Vapillary glucose is never as accurate as venous glucose
NOTE: Patients with T1DM encouraged to check their blood glucose before they inject insulin
What is a continous glucose monitor (CGM)?
It is a device which is inserted subcutaneously to continuously measure glucose levels
- Not as accurate as venous glucose measurements but again good for monitoring trend
How does HbA1c form?
HbA1C = glycated haemoglobin
- Glucose spontaneously binds with Hb in RBCs when present in the bloodstream
- non-enzymatic process
- This binding is initially non-covalent and unstable
- However, over time the bond becomes more stable (covalent, irreversible)
- The amount of this stable bonding is measured in the HbA1C test
What information does HbA1c level tell you?
Glycaemic control over the past 3 months - mainly used for long-term monitoring in diabetic patients as it gives an indication of blood glucose levels over the past 3 months
- Based on the fact that RBC life span = 120 days
- So the higher the blood glucose levels have been, the more glycated Hb should form within the RBCs
Apart from blood glucose levels, what other factors do HbA1C levels depend on?
- Lifespan of red cell (usually about 120 days)
- Rate of glycation (faster in some individuals)
- Haemoglobinopathy, renal failure etc
- Haemoglobiinopathy = reduced or abnormal production of Hb
- Renal failure means lack of erythropoetin so you get reduced RBC production
What are the main acute complications of T1DM?
Rapid decompensation of type 1 diabetes results in:
- Hyperglycaemia:
- Reduced tissue glucose utilisation
- Increased hepatic glucose production
- Metabolic acidosis
- Circulating acetoacetate & hydroxybutyrate → DKA
- Osmotic dehydration due to hyperglycaemia → poor tissue perfusion
- DKA patients are also severely hyperglycaemic so it is common to see dehydration in these patients
Does DKA only occur in patients with T1DM?
DKA predominantly occurs in T1DM
However, it can also occur in T2DM but thsi is much less common
What causes hypoglycaemic episodes (hypos) in diabetes?
Occasional hypos inevitable as a result of treating diabetes
- Major cause of anxiety in patients & families
- Source of major misconceptions in media
Define hypoglycaemia.
Blood glucose < 3.6 mmol/L
Define severe hypoglycaemia.
Any level of hypoglycaemia that requires help another person to treat it
What are the consequences of hypoglycaemia?
- Most mental processes impaired at <3 mmol/l
- Consciousness impaired at <2 mmol/l
- Severe hypoglycaemia may contribute to arrhythmia and sudden death
What can recurrent hypos result in?
Loss of warning (hypoglycaemia unawareness)
-
Usually patients with hypoglycaemia notice that they are hypoglycaemic because they get certain symptoms e.g:
- feeling tired
- tingling lips
- This allows patients to do something to get their blood glucose levels back up again
- However patients with loss of warning may not notice any symptoms until they go into severe hypoglycaemia
NOTE: Hypoglycaemia may have long-term effects on the brain
Who are hypos more common in?
- Main risk factor is quality of glycaemic control
- More frequent in patients with low HbA1c
- Means their blood glucose levels have generally been quite low
At what times during the day do hypos tend to happen?
- Can occur at anytime but often a clear pattern
- Pre-lunch hypos common
- Nocturnal hypos very common and often not recognised
What can trigger a hypo?
- Unaccustomed exercise
- Missed meals
- Inadequate snacks
- Alcohol
- May make you unaware of hypo symptoms
- Liver is working to break down alcohol instead of releasing glucose into the circulation (HGO)
- Inappropriate insulin regime
State some signs and symptoms of hypoglycaemia.
Due to increased autonomic activation:
- Palpitations (tachycardia)
- Tremor
- Sweating
- Pallor / cold extremities
- Anxiety
NOTE:
- Sympathetic activity increases in hypoglycaemia - counter-regulation
- This is because one of the actions of the SNS is to increase blood glucose to provide energy for ‘fight or flight’
- But increases SNS activity this results in all the other effects mentioned above
Due to impaired CNS function:
- drowsiness
- confusion
- altered behaviour
- focal neurology
- coma
How is hypoglycaemia treated?
ORAL - feed the patient
- Glucose
- Rapidly absorbed as solution or tablets
- Complex carbohydrates
- To maintain blood glucose after initial treatment
PARENTERAL - give if consciousness impaired
- IV dextrose e.g 10% glucose infusion
- 1mg Glucagon IM
- Avoid concentrated solutions if possible (e.g 50% glucose)


