Organic Reactions Flashcards
What is this functional group?
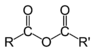
Carboxylic-acid-anhydride
What is this functional group?

Acyl halide
Alcohols
e.g. CH3CH2OH (primary)
CH3CH(OH)CH3 (secondary)
Can be used to synthesise?
Alkenes (CH2=CH2)
Aldehydes (CH3CHO - via primary)
Ketones (CH3COCH3 - via secondary)
Esters (CH3COOCH2CH3)
What is this functional group?

Keytone
Ketones
e.g. CH3COCH3
Can be used to synthesise?
Alcohols (CH3CH(OH)CH3)
Haloalkanes
CH3CH2Br
CH3CHBrCH3
can be used to synthesise?
Amines (R-CH2NH2)
Nitriles (R-CN)
Alcohols (R-OH)
All via Nucleophilic Substitution
Alkanes (CH3CH3)
via Elimination
Aldehydes
e.g. CH3CHO
Can be used to synthesise?
Carboxylic Acids (CH2=CH2)
Alcohols (CH3CH2OH)
Nitriles
e.g. R-CH2-CN
Can be used to synthesise?
Amines (CH3CNH2)
via Reduction
Alkanes
e.g. CH4
can be used to make?
Haloalkanes
e.g CH3Cl, CH2Cl2, etc.
Via free-radical substitution with Cl2 (UV light)
What is this functional group?
(A & B may be C, O, H or N)

Carbonyl Group
Carboxylic Acids
e.g. CH3COOH
Can be used to synthesise?
Esters (CH3COOR)
Esters
e.g. CH3COOR
Can be used to synthesise?
Carboxylic Acids + Alcohol
CH3COONa + ROH
Alkenes
e.g. CH2=CH2
Can be used to synthesise?
Alkanes (CH3CH3)
Haloalkanes (CH3CH2Br)
Di-haloalkanes (CH2BrCH2Br)
Alcohol (CH3CH2OH)
Polymers -[CH2-CH2-]n










