Organic Chemistry Flashcards
carbon-carbon sigma bonds
list all possibilities strong to weak (6)
identify hybridisation (ie how many p)
identify shortest bond length
draw examples
strongest has most s character and least p
(similar to why sigma bonds are stronger than pi bonds)
137 Angstroms
sp-sp 2p
sp-sp2 3p
sp-sp3 4p
sp2-sp2 4p
sp2-sp3 5p
sp3-sp3 6p
154 Angstroms

Structural formulae
List and describe possible structural formulae (7)
- Lewis dot structure
- Dash formula - shows the bonds between atoms but not their 3D structure H-C-C..
- Condensed formula - CH3CH2…
- Bond line formula - intersections, corners and endings are assumed to be C unless other drawn in. H not drawn
- Fischer projection - vertical lines into page, horizontal out of page
- Newman projection - view straight down axis of one of the sigma bonds. Circle and intersecting lines are C
- Dash-line-wedge model - wedge out of page, dashed into
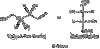
Structures
Draw
n-propyl
isopropyl
n-butyl
isobutyl
sec-butyl
tert-butyl
Straight carbon chain naming
The general formula for a alkane is …
The general formula for an alkene is …
The general formula for an alkyne is …
The general formula for an alkane is Cn H2n+2
The general formula for an alkene is Cn H2n
The general formula for an alkene is Cn H2n-2
In organic chemistry, a saturated compound has ….
In organic chemistry, a saturated compound has no double or triple bonds
Saturated compounds
Describe saturated and unsaturated carbon compounds
Full complement of hydrogens = saturated.
In organic chemistry, a saturated compound is a chemical compound that has a chain of carbon atoms linked together by single bonds. Alkanes are an example of saturated compounds.
An unsaturated compound is a chemical compound that contains carbon-carbon double bonds or triple bonds, such as those found in alkenes or alkynes, respectively. Saturated and unsaturated compounds need not consist only of a carbon atom chain. They can form straight chain, branched chain, or ring arrangements. They can have functional groups, as well.
In other unsaturated hydrocarbons, the double bond between two carbons prevents rotation of the atoms about the bond, locking them into specific structural formations. When attached atoms occupy similar positions on each carbon, they are referred to as “cis”, and when they are on opposite sides, they are called “trans”. Most natural hydrocarbons exist in the cis state, but artificially manufactured hydrocarbons are trans. The body lacks the enzymes to properly break down the trans configuration. This is why trans fats are viewed as dangerous and unhealthy, as they tend to build up. Unsaturated compounds of the two formations are classified as geometric isomers of one another.
Electron pushing arrows
- What are they and where are used?
- What are the parts of the arrow and what do they mean?
- Between two atoms what are the three possible combinations for an electron?
- How can you move between these combinations?
- They show electron movement from one atom to another.
- Electron moves from tail to head(>)
- Own eg a lone pair completely owned by an atom
Share eg a covalent bond
Lack eg a lone pair on a completely different atom
- One step at a time
Own Share Lack
Functional groups - nomenclature
Give the prefix and suffix of the
- Ether
- Ether - alkoxy - ane
- Aldehyde -formyl -al (-carbaldehyde)
- Ketone -oxo -one
- Ester (R)-oxycarbonyl -oate
- Anhydride -oic ** -anhydride
- Epoxide -epoxy -oxide
** eg propanoic anhydride (for symmetrical) or propanoic ehtanoic anhydride
Functional groups - nomenclature
Give the prefix and suffix of the following functional group
- Benzene ring
Functional groups - nomenclature
Give the prefix and suffix of the following functional groups
- Benzene ring -phenyl -benzene
- Enol -?? (E) … -ol
- Enoloate
Functional groups - nomenclature
Give the prefix and suffix of the following functional groups
- Amine
Functional groups - nomenclature
Give the prefix and suffix of the following functional groups
- Amine -amino -amine
- Nitro -nitro -(always substituent)
- Nitrile -cyano -nitrile
- Imine -?? -imine
- Amide -carbonyl -carboxamide(amide)
- Enamine -?? -??
Functional groups - nomenclature
Give the prefix and suffix of the following functional groups
- Acyl halide
Functional groups - nomenclature
Give the prefix and suffix of the following functional groups
- Alkyl halide -haloalkyl -ane
- Acyl halide -n/a -oyl halide
(2-methylpropanoyl chloride)
Electron pushing arrows
- What is the most important thing in organic chemistry?
- What are the valid charges in organic chemistry (at least in org 1 and 2)?
- How do electron pushing arrows tell us to break a bond?
- The charges
- -1 0 +1
- The tail is on the bond
Functional groups - nomenclature
Give the prefix and suffix of the following functional group
- Epoxide
- Ether - alkoxy - ane
- Aldehyde -formyl -al (-carbaldehyde)
- Ketone -oxo -one
- Ester (R)-oxycarbonyl -oate
- Anhydride -oic ** -anhydride
6. Epoxide -epoxy -oxide
** eg propanoic anhydride (for symmetrical) or propanoic ehtanoic anhydride
Functional groups - nomenclature
Give the prefix and suffix of the following functional group
- Anhydride
- Ether - alkoxy - ane
- Aldehyde -formyl -al (-carbaldehyde)
- Ketone -oxo -one
- Ester (R)-oxycarbonyl -oate
5. Anhydride -oic ** -anhydride
- Epoxide -epoxy -oxide
** eg propanoic anhydride (for symmetrical) or propanoic ehtanoic anhydride
Functional groups - nomenclature
Give the prefix and suffix of the functional group
- Ester
- Ether - alkoxy - ane
- Aldehyde -formyl -al (-carbaldehyde)
- Ketone -oxo -one
- Ester (R)-oxycarbonyl -oate
- Anhydride -oic ** -anhydride
- Epoxide -epoxy -oxide
** eg propanoic anhydride (for symmetrical) or propanoic ehtanoic anhydride