Molecular Shape and Polarity Flashcards
Name shapes and bond angles given lewis structures. Then link to polar or non-polar based on symmetry and bond type
Shape and bond angle?
Polar or non-polar?

Linear, 180o
Polar, symmetric, different bonds
Shape and bond angle?
Polar or non-polar?

Linear, 180o
Non-polar, symmetric, same bonds
Shape and bond angle?
Polar or non-polar?

T-Shape 90o and 180o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?

T-Shape 90o and 180o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?
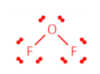
Bent, 109.5o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?

T-Shape 90o and 180o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Square planar 90o
Non-polar, symmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Square pyramid 90o and 180o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Trigonal pyramid, 109.5o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Trigonal planar 120o
Polar, symmetric, different bonds
Shape and bond angle?
Polar or non-polar?

Trigonal pyramid 109.5o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?

See-Saw 90o, 120o and 180o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Trigonal pyramid, 109.5o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Linear 180o
Non-polar, symmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Octahedral 90o
Non-polar, symmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Trigonal planar, 120o
Non-polar, symmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Bent, 120o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Square planar 90o and 180o
Non-polar, symmetric, same bonds
Shape and bond angle?
Polar or non-polar?

T-Shape 90o and 180o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Trigonal bipyramid 120o and 90o
Non-polar, symmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Tetrahedral 109.5o
Non-polar, symmetric, same bonds
Shape and bond angle?
Polar or non-polar?

See-Saw 90o, 120o and 180o
Polar, asymmetric, different bonds
Shape and bond angle?
Polar or non-polar?

Bent, 109.5o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Square pyramid 90o and 180o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Tetrahedral, 109.5o
Non-polar, symmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Square pyramid 90o and 180o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Octahedral 90o
Non-polar, symmetric, same bonds
Shape and bond angle?
Polar or non-polar?

See-Saw 90o, 120o and 180o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Trigonal bipyramid 90o and 120o
Polar, asymmetric, same bonds
Shape and bond angle?
Polar or non-polar?

Octahedral 90o
Non-polar, symmetric, same bonds


