Mod VI: Pain Pathway Flashcards
Pain Pathway
Two mechanism:
Excitatory Mechanisms = Ascending pathway
Inhibitory Mechanisms – Descending pathway
Pain Pathway
Excitatory Mechanisms = Ascending pathway
Physiological pain is mediated by a sensory system consisting of primary afferent neurons, spinal interneurons, ascending tracts, and supraspinal areas
Trigeminal and dorsal root ganglia (DRG) give rise to high-threshold Aδ– and C-fibers innervating peripheral tissues (skin, muscles, joints, viscera)
These specialized primary afferent neurons (nociceptors) transduce noxious stimuli into action potentials and conduct them to the dorsal horn of the spinal cord
When peripheral tissue is damaged, primary afferent neurons are sensitized and/or directly activated by thermal, mechanical, and/or chemical stimuli
Impulses are transmitted to spinal neurons, brainstem, thalamus, and cortex
Repeated nociceptor stimulation can sensitize peripheral and central neurons (activity-dependent plasticity, or “wind-up”)
This can be sustained by changes in the expression of genes coding for various neuropeptides, transmitters, ion channels, receptors, and signaling molecules (transcription-dependent plasticity) in peripheral and central neurons (Baron, Hans, & Dickenson, 2013; Basbaum et al., 2009)
Both induction and maintenance of central sensitization are critically dependent on the peripheral drive by nociceptors, indicating that therapeutic interventions targeting such neurons may be particularly effective, even in chronic pain syndromes (Baron et al., 2013; Richards & McMahon, 2013).
Pain Pathway
Inhibitory Mechanisms – Descending pathway
Concurrent with such excitatory events, powerful endogenous mechanisms counteracting pain unfold
This was initially proposed in the “gate control theory of pain” of 1965 and has since been corroborated and expanded by experimental data in the central nervous system (CNS) and in the periphery
In 1990, a “peripheral gate” was discovered at the source of pain generation by demonstrating that immune cell–derived opioid peptides can block the excitation of nociceptors carrying opioid receptors within injured tissue (Figure 1) (Stein et al., 1990)
This represented the first example of many subsequently described neuro-immune interactions relevant to pain (Machelska, 2011; Stein, 1995; Stein & Machelska, 2011)
In the spinal cord, pain inhibition is mediated by the release of opioid peptides, gamma-amino-butyric acid (GABA), or glycine
During ongoing nociceptive stimulation, spinal interneurons upregulate gene expression and production of opioid peptides (Herz, Millan, & Stein, 1989)
Powerful descending inhibitory pathways from the brainstem also become active by operating through noradrenergic, serotonergic, and opioid systems (Basbaum et al., 2009; Schumacher, Basbaum, & Naidu, 2015)
The supraspinal integration of signals from excitatory and inhibitory neurotransmitters, and cognitive, emotional, and environmental factors eventually results in the central perception of pain
Terminologies - Acute and Chronic Pain - Basic Concepts
Pain may be divided into two broad categories:
Physiological & Pathological pain
Physiological=acute
Pathological=chronic
Terminologies - Acute and Chronic Pain - Basic Concepts
Physiological pain = acute pain
Nociceptive pain is a warning sign that usually elicits reflex withdrawal and thereby protects from further injury
=> POSTOPERATIVE PAIN
Terminologies - Acute and Chronic Pain - Basic Concepts
Pathological pain = chronic pain
Neuropathic pain is an expression of the maladaptive operation of the nervous system
It is “pain” as a disease
Terminologies - Acute and Chronic Pain - Basic Concepts
Non-malignant chronic pain is frequently classified into
Inflammatory (e.g., arthritic)
Musculoskeletal (e.g., low back pain), headaches
Neuropathic pain (e.g., post-herpetic neuralgia, phantom pain, complex regional pain syndrome, diabetic neuropathy, HIV neuropathy)
Terminologies - Acute and Chronic Pain - Basic Concepts
Malignant pain is related to
Cancer and its treatment
Cancer pain can originate from the invasion of the tumor into tissues innervated by primary afferent neurons (e.g., pleura, peritoneum) or directly into peripheral nerve plexus
In the latter case, neuropathic symptoms may be predominant
Could turn into neuropathic pain
Acute and Chronic Pain - Clinical Concepts: Definitions and Prevalence
“an unpleasant sensory and emotional experience associated with actual or potential tissue damage” is the definition of:
Pain
<strong>[</strong>The International Association for the Study of Pain (IASP)]
Pain is always a psychological state, even though it often has a proximate physical cause
Acute and Chronic Pain - Clinical Concepts: Definitions and Prevalence
The neurophysiological activity in peripheral sensory neurons (nociceptors) and higher nociceptive pathways is
Nociception
is defined as the “neural process of encoding noxious stimuli.”
Nociception is not synonymous with pain
Pain is always a psychological state, even though it often has a proximate physical cause
Acute and Chronic Pain - Clinical Concepts: Definitions and Prevalence
When the intricate balance between biological (neuronal), psychological (e.g., learning, memory, distraction), and social (e.g., attention, reward) factors becomes disturbed, what type of pain develops?
Chronic pain
Chronic pain has enormous socioeconomic costs due to health care, disability compensation, lost workdays, and related expenses.
According to a report in 2011, by the Institute of Medicine titled: Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research, pain is a significant public health problem that costs society at least $560-$635 billion annually, an amount equal to about $2,000.00 for everyone living in the U.S.
This includes the total incremental cost of health care due to pain from ranging between $261 to $300 billion and $297-$336 billion due to lost productivity (based on days of work missed, hours of work lost, and lower wages)
Acute and Chronic Pain - Clinical Concepts: Definitions and Prevalence
Today, pain’s impact on society is still great, and indeed what are the number one reason patients seek medical advice
Pain complaints
(http://pharmacistsalternative.com/uncategorized/pain-part-1/)
Acute and Chronic Pain - Bio-psycho-social Concept
Both cancer and non-cancer patients with chronic pain have in common the complex influences of which factors?
Biological (tissue damage)
Cognitive (memory, expectations)
Emotional (anxiety, depression)
Environmental factors (reinforcement, conditioning)
Acute and Chronic Pain - Bio-psycho-social Concept
Pain behaviors such as limping, medication intake, or avoidance of activity are all subject to operant conditioning; that is, they respond to
Reward and punishment
For example, pain behaviors may be positively reinforced by attention from a spouse or healthcare provider (e.g., by inadequate use of medications)
Conversely, such behaviors can be extinguished when they are disregarded or when incremental activity is reinforced by social attention and praise
The interplay between biological, psychological, and social factors results in the persistence of pain and illness behaviors
Besides possible long-term neuronal sensitization, this concept helps us understand why chronic pain may exist without obvious physical cause.
Acute and Chronic Pain - Pain Management
The treatment of both acute (e.g., postoperative) and chronic pain remains a major challenge in clinical medicine and public health
One component of pain therapy is the use of analgesic drugs - How do these drugs work?
They interfere with the generation or transmission of impulses in the periphery or CNS meaning nociception
Acute and Chronic Pain - Pain Management
Drugs currently used in clinical pain treatment include
Opioids
Nonsteroidal anti-inflammatory drugs (NSAIDs)
Serotonergic compounds
Antiepileptics, and
Antidepressants
Acute and Chronic Pain - Pain Management
In chronic pain, treating only nociception is obviously insufficient - why?
A bio-psycho-social approach addresses physical, psychological, and social skills and underscores the patients’ active responsibility to regain control over their life by improving their function and well-being
Acute and Chronic Pain
Types of pain include:
- Nociceptive Pain
- Somatic Nociceptive Pain
- Visceral Nociceptive Pain
- Referred Visceral Pain
- Hyperalgesia
- Allodynia
- Neuropathic Pain
- Hyperesthesia
Types of Pain
Painful stimulus that causes an organism to adopt protective behaviors that promote healing - Pain with a well defined onset associated with tissue injury from surgery, trauma, or disease related injury including inflammation - These characteristics of which type of pain?
Nociceptive pain
Types of Pain
What are the phases of Nociceptive pain?
Transduction
Transmission
Perception
Modulation
Types of Pain
Pain that is often described as well-localized sharp, crushing, tearing pain that usually follows a dermatomal pattern
Somatic nociceptive pain
Types of Pain
Pain that is poorly localized dull, cramping, or colicky pain associated with peritoneal irritation, dilation of smooth muscle or a tubular passage
Visceral nociceptive pain
Types of Pain
Pain that radiates in a somatic dermatomal pattern. Example MI
Referred visceral pain
Types of Pain
Which physiologic change results from prolonged hyper-stimulation which can cause structural and functional changes to both peripheral and central neurons
HyperalgesiaThese changes can cause central sensitization leading to the development neuropathic pain
Types of Pain
Normally, nociceptor terminals have a high activation threshold
They requiring intense stimulation to generate an Ascending pathway
For example, thermal nociceptors are only activated by temperature extremes (>45°C or < 5°C)
However, nociceptors can be made more sensitive to stimuli
Injury to neurons and surrounding tissues expose neighboring nociceptors to irritating substances, including: neurotransmitters, ATP, prostanoids, bradykinin, serotonin, histamine, and hydrogen ions (acid pH), etc.
These substances lower the nociceptor’s activation threshold (sensitize), creating a condition of
Hyperesthesia
Hyperesthesia is a term that encompasses both
allodynia and hyperalgesia
A common example of allodynia is the painful response to touch in an area of a 1st degree burn, e.g. sunburn
Normally, allodynia subsides as healing progresses
Often you will hear Allodynia in association with Multiple Sclerosis
Types of Pain
Hyperesthesia is a term that encompasses both
Allodynia & Hyperalgesia
Types of Pain
the painful response to touch in an area of a 1st degree burn, e.g. sunburn is a common example of:
Allodynia
Normally, allodynia subsides as healing progresses
Often you will hear Allodynia in association with Multiple Sclerosis
Opioids
Exogenous opioids like hydromorphone, morphine and oxycodone produce analgesia by
mimicking endogenous endorphins
Opioids
Opioids are able to activate which endorphin receptors?
Mu, Kappa & Delta

Opioids
Which opioid receptors are responsible for most of the analgesic effect of opioids and are present on neurons in the spinal cord, brainstem and midbrain?
Mu receptors
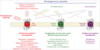
Opioids
The opioid receptor family contains three pharmacologically distinct receptors, named
Mu-, delta-, and kappa
(MOR, DOR, KOR)

Opioids
Which type of receptors are Opioid receptors (OR)?
Opioid receptors (OR) are G protein–coupled receptors (GPCR) that are the physiological targets of endogenous opioid peptides

Opioids
Endomorphins and endogenous morphine and exogenous opioids like morphine, fentanyl, oxycodone show selectivity for which receptors elicit analgesia and other effects?
Mu receptors

Opioids
What’s the MOA of Full agonist opioids? What are examples of Full agonist opioids?
Activate the opioid receptors in the brain fully resulting in the full opioid effect
Examples of full agonists are heroin, oxycodone, methadone, hydrocodone, morphine, opium and others
Opioids
What’s the MOA of opioid antagonists? What are examples of opioid antagonists?
An antagonist is a drug that blocks opioids by attaching to the opioid receptors without activating them
Antagonists cause no opioid effect and block full agonist opioids
Examples are naltrexone and naloxone
Naloxone is sometimes used to reverse a heroin overdose
Opioids
Buprenorphine is classified as:
Partial agonist at mu and kappa opioid receptors and as an antagonist at delta receptors.
Buprenorphine is a derivative of the opioid alkaloid thebaine that is a more potent (25 - 40 times) and longer lasting analgesic than morphine
It acts as a partial agonist at mu and kappa opioid receptors and as an antagonist at delta receptors

Opioids
Nubain is classified as:
kappa agonist and partial mu and delta antagonist
(see in OB)

Pain Pathway
Most of us in our lifetime may have done something that lead to a painful situation occurring in our bodies - Whether it’s an injury, surgery, illness or disease an uncomfortable sensation may be experienced and potentially perceived as pain - The sensations of pain: pricking, burning, aching, stinging and soreness are the most distinctive of all the sensory modalities - While pain can serve as a warning for protection against further harm, however unmanaged it can also lead to severe and relentless suffering - Pain is manifested by autonomic, psychological and behavioral reactions - The clinical word for pain perception is:
Nociception
Pain Pathway
The body recognizes pain when some sort of stimulus causes a signal to be sent through the nervous system and into the brain
The stimulus can be for instance,
mechanical, such as a puncture from a needle,
chemical, like a burn or a chemical irritation or
thermal
Pain Pathway
Pain stimuli are transformed into […], which are then conducted to the central nervous system.
electrical signals
Pain Pathway
[—] are slow, thin, myelinated fibers associated with sharp/pricking, well localized pain.
A-delta fibers (Að)

Pain Pathway
[—] are very slow, thin, unmyelinated fibers that are associated with a dull, aching, throbbing, diffuse pain.
C fibers

Pain Pathway
There have been three types of nociceptors identified [—], which are free nerve endings
Aδ, Aβ, C-fibers (primary afferent fibers)

Pain Pathway
Which fibers carry electrical signals towards the central nervous system?
Afferent fibers

Pain Pathway
Highly myelinated and of large diameter fibers, therefore allowing for rapid signal conduction. They have a low activation threshold and usually respond to light touch and transmit non-noxious stimuli.
Aβ fibers

Pain Pathway
Lightly myelinated and smaller diameter fibers, and hence conduct more slowly. They respond to mechanical and thermal stimuli. They carry rapid, sharp, pricking pain and are responsible for the initial reflex response to acute pain
Aδ fibers

Pain Pathway
Unmyelinated and are also the smallest type of primary afferent fibers. Hence they demonstrate the slowest conduction. Are polymodal, meaning they respond to chemical, mechanical and thermal stimuli. Activation leads to slow, burning, long lasting pain.
C-fibers

Pain Pathway
THE MAJOR INHIBITORY NEUROTRANSMITTER IN THE NERVOUS
SYSTEM is:
GABA
Aminobutyric acid (GABA) is the major neurotransmitter for fast
inhibitory synaptic transmission
The GABA-A receptor is a chloride channel regulated by GABA binding, and it is now grouped in the superfamily of ligand-gated ion channel receptors, mediated by a G-protein–coupled receptor that increases potassium conductance
Pain Pathway
What’s the major neurotransmitter for fast excitatory synaptic transmission?
Glutamatel-Glutamic acid (glutamate) is the major neurotransmitter for fast excitatory synaptic transmission
Excitatory neurotransmitters are Glutamate and substance P
























