Mechanisms of neuronal death Flashcards
Mechanisms of Ca++ mediated neuronal loss
- *Genaration of ROS via:**
- mitochondrial dysfunction
- nitric oxide synthase
- phospholipase A2 activation
**Stimulation of proteases.
Activating of stress activated protein kinases**
Activation of endonucleases. Degradation of DNA, compromising the cell and leading to neuronal cell death
Formation of caspases and apoptosis
How does arachidonic acid mediate excitotoxicity?
Positive feedback cycle with glutamate
- Increases in levels of cytosolic calcium binds to cytosolic PLA2 which is transported to the membrane, where it liberates AA from phosphotidiyl inositol
- High concentrations of AA activate the NMDA receptor
- High concentrations of AA block GLT-1 transporter on astrocytes, increasing the level of glutamate in the synaptic cleft.
- AA in the presynaptic cell can activate PKC, leading to more glutamate release.
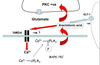
How does nitric oxide mediate excitotoxicity?
positive feedback cycle
- Glutamate stimulates the production of nitric oxide by nNOS.
- NO diffuses to the presynaptic cell where it activates guanylyl cyclase
- Product of cGMP by guanylyl gyclase leads to increased glutamate production
It is also produced by microglia by inducible iNOS during inflammation
It can combine with superoxide to form perioxynitrite
What role may iron play in neurodegenaration?
The fenton reaction: H2O2 + Fe2+ = OH. + OH- + Fe3+
Hydrogen peroxide reacts with iron to create a hydroxyl free radical.
Iron accumulates in our brains as we age, and may accelerate cell death through free radical formation.
How do free radicals cause protein misfolding?
By reacting with residues such as cystein residues.
This is also a normal part of signalling, as it allows protein-protein interactions. Redox recycling is critical.
Ryanodine senses how many free radicals are being produced and maintains their equibilrium. Mice overexpressing amyloid and KO for ryanodine make even more amyloid.
Why are MAPKs a poor therapeutic drug target?
They play part of stress activated response pathways, and therefore would be useful to antagonise. However, many MAPKs have a T-X-Y sequence in their active sites catalytic core that is shared with many other proteins, some of which are important for growth and proliferation. It is therefore difficult to get a drug specific to beneficial pathways.
What is the JNK signalling pathway?
It mediates O.S to neuronal stress.
It is triggered by oxidative stress or lipid peroxidation.
It leads to programmed upregulation of cell death genes such as FAS-L and activates caspases.
What are JIP proteins?
They are cellular scaffolding proteins that bring together proteins involved in signalling pathways so that they can activate one another.
JIP3 is involved in cell death signalling.
KO could be a target but it is very difficult to knock them out and can lead to detrimental effect.
Apoptosis
programmed cell death, characterised by an ordered disassembly of cell components, membrane blebbing and cell shrinkage.
Necrosis
Uncontrolled cell lysis
What are the other cell death pathways besides apotosis and necrosis?
There are other regulated cell death pathways: Necroptosis, PARP1, Autophagy
What is the intrinsic pathway of apoptosis?
- Pro-apoptotic signals induce formation of mitochondrial permeability transition pore (mitochondria made permable to proteins < 1500KD) and stimulate the relase of cytochrome c from mitochondria
- Cyt c activates caspases (cysteinyl aspartate specific proteases)
- Caspases (3-9) regulate apoptosis. However caspase three is also essential to neurite outgrowth
What is the extrinsic pathway of apoptosis?
membrane death receptors and Caspase 8


