Mass Spectrometry Flashcards
How do you know if you’ll be able to distinguish the difference between compounds depending on resolution?
Find the difference in ppm and see if it is higher than resolution

How do you use relative abundance to find intensities of peaks
If F or I then ignore
If Cl or Br then consider how many there are and use binomial expansions to find it
Explain alpha cleavage
When lp becomes a radical then cleavage is possible at the alpha site

Explain the mclaferty rearrangment
hydrogens gamma to carbonyl can be exchanged. This causes a subsequent c cleavage

Explain how H rearrangment takes place in the cleavage of a cyclic molecule.
Alpha cleavage. Resulting radical can exchange a proton neaby before radical performs a carbon cleavage

What’s something special about an odd m/z
Compound likely contains nitrogen
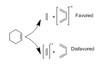
For a reverse diels alder reaction, which molecules will be charged and be radicals if EI is used?

The diene is favored due to higher conjugation and stability
For the loss of
1, 15, 29, 31, 43 and 45 what
What are possible leaving groups?
1 = H
15 = CH3
29 = .CH2CH2CH3 or .CHO
31 = .OMe
43 = .CH2CH2CH2CH3
45 = Ac. or CO2H
For the loss of
18, 28, 44, 90
What are possible leaving groups?
18 = H2O
28 = CO
44 = CO2
90 = HO-Si-(CH3)3
What is the natural abundance and molecular mass of all isotopes of Br?
Br, 79 & 81
50%, 50% abundance respectively
What is the natural abundance and molecular mass of all isotopes of F?
F, 19
100%
What is the natural abundance and molecular mass of all isotopes of I?
I, 127
100%
What is the natural abundance and molecular mass of all isotopes of Cl?
Cl, 35, 37
75% and 25% respectively

