Magnetism Flashcards
Give the equation for M, the magnetisation of a material in a magnetic field H
Xm is the magnetic susceptibility, ie how magnetic the material is per unit volume

Give the equation for the magnetic susceptibility per gram
Note that p is the density of the material

Give the equation for the molecular susceptibility
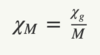
Define diamagnetism
A diamagnetic material is one which tends to repel the applied field, ie forms a magnetic field in the opposite direction to the one applied. Diamagnetism arrises due to the circulation of electron pairs within an atom. All electrons are paired up.
χ < 0 for a diamagnetic material
Define paramagnetism
When samples are placed in a magnetic field, you may find that they are magnetised in the same direction as the applied field. This is called paramagnetism and is due to the material containing unpaired electrons - radicals.
State and describe the Curie law
Spins in a material are subject to thermal motion. The magnitude of magnetic susceptibility, therefore, depends on temperature.
Pierre Curie discovered that for paramagnetic materials, X is inversely proportional to T
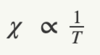
Describe a material if χ > 0
For any material, its core electrons will exhibit diamagnetic behaviour, and any unpaired valence electrons will exhibit paramagnetic behaviour.
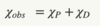
Describe how to count unpaired electrons
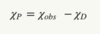
Describe the magnetic moment μeff
μeff tells us how strong each individual molecular magnet is

Give the spin only formula
S is the spin quantum number
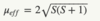
Give the spin only formula using n instead of S
Where n is the number of unpaired electrons
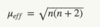
Give the three conditions for an orbital contribution to the magnetic moment to be observed for the first row of transition metals
- You must have degenerate orbitals or orbitals of very similar energy
- There must be an electronic degeneracy - you could have the same number of unpaired electrons but arranged in different orbitals
- The orbitals must have similar shapes but different orientations so that when you move from one to the other you get a rotation around an axis


