lecture 26: breast and cancer stem cells Flashcards
What defines stem cells?
- defined by their ability to self-renew and differentiate along multiple lineages
- stem cell → common progenitor → committed progenitor → mature cells
- stem cell → stem cell

What is development of the breast?
- newborn → puberty → pregnancy → lactation → involution (→ pregnancy → etc)
- proliferation at puberty
- proliferation and differentition during pregnancy
- differentiation during lactation
- apoptosis during involution

What are the three distinct epitheli cell types seen in breast?
- 18 day pregnant
- luminal epithelium
- alveolar
- ductal
- myoepithelium

For what are mammary stem cells required?
- homeostasis in the mammary gland and growth during pregnancy
- remarkable generative capacity of breast tissue
- more than 25-fold expansion of breast ‘epithelium’

How have in vivo strategies been used to define the mammary stem cell (MaSC)?
- identification of cell surface markers to allow fractionation of mammary cells by flow cytometry
- transplantation studies:
- perform limiting dilution assays to allow comparison of the relative repopulating frequencies of different subpopulations
- demonstrate the multilineage differentiation capacity of SCs. Serial transplantation is the ‘gold standard’ to prove the self-renewing capability
- lineage tracing studies in vivo
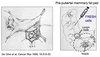
What is in vivo characterization using mammary fat pad transplantation?
- mammary gland not an essential organ
- cauterize in a young animal (3 weeks)
- remove rudimentary tree attached to nippple
- leave behind an intact fat pad
- inject FRESH cells into this
- harvest 8 weeks post-transplantation
- ask whether we see a ductal tree that has emerged in this area
What are the multiple cell types of the mammary gland?
- luminal epithelial cells
- myoepithelial cells
- basement membrane (separates epithelial cells from surrounding stroma)
- fibroblasts
- adipocytes
- blood vessel
- lymph node
- macrophages
- complex microenvironment

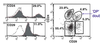
How many epithelial subpopulations were defined by cell surface markers?
- CD24 + or -
- heat stable antigen
- CD29 + or -
- beta1-integrin
- DP - double positive - smallest population, 4.8%
- CD24+/CD29- 23.9%
- double negative = 55.4%
- CD29+/CD24- = 5/3%
- transplant cells in numbers proportional to their frequency in the overall population
What was transplantation of subsets of lineage-cells?
- cells double-sorted
- rosa 26 donors (lacZ gene in Rosa-26 locus)
- only one population had repopulating capacity when put into cleared fat pad
- CD29hi
- CD24+

Were they able to get generation of a functional mammary gland from a single stem cell?
- yes
- beta-galactosidase makes blue
- capable of multilineage differentiation

Can the Lin-CD24+CD29hi cell self-renew?
- serial transplantation studies
- MaSC
- primary transplantation
- primary outgrowth
- secondary transplantation ( first generation self-renewed MaSCs)
- secondary outgrowth
- tertiary transplantation (second gen self-renewed MaSCs)
- tertiary outgrowth
- most could be passaged ~8 times

Do bipotent cells exist and function in vivo?
- big controversial issue in the field
- they had done research with a team in canada → recapitulated findings independently → found a stem cell that could give rise to all of these cells
- in 2011 a paper appeared and said that bipotent stem cells do not exist - only unipotent cells exists
- there is a myo-SC → myoepithelial cells
- luminal SC → ductal and alveolar cells
- claimed using lineage tracing
- at the same time they were also carrying out lineage tracing experiments

What is 3D imaging of the mammary gland?
- 3 week-old gland prior to puberty
- this is about a 4mm section of an intact mammary gland
- can see elongated myoepithelial cells

What is the strategy for in vivo lineage tracing?
- Rosa26 is a strong, ubiquitous promoter
- dtTomato is a red fluorescence protein
- all daughter cells of labeled parental cell are permanently marked
- single colour for quantification
- toxicyclin inducible system
- tet operon that contains cre
- third cross where cre induces expression of a promoter gene
- triple transgenic mice
- if the promoter is expressed in a specific cell of interest, in the presence of toxicyclin, it will activate the tet operon → cre mediating recombination → expression of that particular promoter or reporter gene in that particular cell type
- indelible marking of all daughter cells of that parental cell

What is confetti?
- a stochastic multicolour cre reporter for clonality studies
- 4 fluorescent proteins
- if you deliver just a small single pulse of an agent (toxicyclin) → random activation of only one of four colours in that cell
- because only one pulse is delivered not flipping backwards and forwards

What was population dynamics of K5-expressing cells in puberty?
- equi-expression of all four fluorescent proteins
- K5 marks long myoepithelial cells
- shows that many progenitor cells are involved in morphogenesis of the gland during puberty

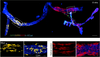
What were they able to show in the end?
- K5 marks both luminal and myoepithelial cells after induction in the adult
- able to show that after an 8 week chase
- unicoloured clonal regions
- both myoepithelial and cuboidal luminal
- proved that bipotent stem cells exist
- capable of giving rise to all the epithelial cells of the mammary gland
- also luminal progenitor cell exists
What is the search for normal human breast stem cells?
- not able to do lineage tracing but did do cell surface marker analysis
- found that there were four distinct populations
- only one of these, if you transplant back into the mouse fat pad, has the ability to give rise to ductal outgrowths
- same pattern for human and mouse mammary tissue

What are functional similarities between mammary epithelial subpopulations in mouse and human?
- can identify bipotent stem cells in both
- two types of luminal progenitors
- can prospectively isolate all the mature cells as well
- don’t know about all the precursors leading up to myoepithelial cells
- both the mouse and human MaSCs lack receptors for the ovarian hormones oestrogen and progesterone
- important because increased progesterone and oestrogen are linked with increased breast cancer risk
- could they still be influenced?
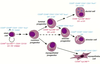
Are MaSCs sensitive to ovarian hormone deprivation?
- yes - highly sensitive
- stem cells appear to retain a ‘memory’ of prior steroid hormone deprivation
- repopulating frequency:
- control: 1/58
- ovariectomy: 1/247
- also when they could generate tissue, it was only very little
What happens to numbers of MaSCs during pregnancy?
- pregnancy is accompanied by an 11-fold increase in the number or activity of MaSCs
- the augmented MaSC pool drives secretory cell expansion
- seen in mid-pregnancy
- transplantation assay measures function
- stem cell highly receptive to hormonal signalling

What is evidence that ovarian hormones (oestrogen and progesterone) profoundly influence stem cell activity?
- hormone deprivation and anti-oestrogens decrease MaSC pool/activity
- excess hormones increase MaSC function
- ageing is associated with increased MaSC function
- pregnancy dramatically increases MaSC pool
- ductal luminal cells express ER and PR - remain the most important prognostic markers for breast cancer to date
- 70% of breast cancers are ER positive and if they are there is a much better prognosis
- how is this signalling to the stem cell when the stem cell doesn’t have receptors for the hormones?

What pathway mediates steroid hormone signalling to stem cells?
- The RANKL/RANK pathway
- signals to NFkB
- steroid hormones like progesterone stimulate ductal cell to make RANKL
- signals to the stem cell pool
- drives proliferation
- probably other molecules involved as well

What is a strategy for assessing gene function in mammary stem and progenitor cells?
- retrovirus-mediated manipulation of gene expression in discrete populations
- harvest virgin mammary gland
- single cell suspension
- cell sorting into subpopulations
- plate subpopulation on feeder cells
- transduce with retrovirus
- sort GFP+ cells after single cell suspension
- colony forming assay on feeder cells
- colony forming assay in matrigel
- transplant into cleared fat pad
- puberty or pregnancy
- mammary outgrowth
What are pathways often deregulated in cancer?
- self-renewal pathways in stem cells
- decided to look at notch - associated with the most aggressive subtype of cancer (triple negative)
- wanted to understand the normal role of notch
- wnt, notch and hedgehog are all very important pathways → embryonic and normal development
- always see them reemerging in cancer

Can short-term cultured MaSCs reconstitute a functional mammary gland?
- yes
- 5 or 6 days of culture did not abrogate this ability

What does constitutive Notch signalling in MaSCs promote?
- luminal cell commitment and induces hyperplasia in vivo
- deregulated notch1 can go on to produce luminal tumours

What are dual functions for Notch?
- restricts MaSC proliferation and directs luminal cell fate determination
- complex pathway
- normally it represses proliferation of the mammary stem cell
- but when activated it promotes formation of luminal progenitors
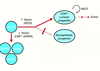
What are GATA transcription factors?
- six family members (GATA-1-GATA-6)
- bind DNA at the consensus sequence (A/T)GATA(A/G)
- play critical roles in development, cell-fate specification and differentiation
What is gata-3?
- gata-3 is a master regulator of mammary gland development in the embro and adult
- in the embryo:
- essential for placode formation (E11.5) → bud (E13.5) → nipple sheath (E16.5)
- in puberty:
- important for development of the tree
- pivotal for differentiation into a milk producing cell
- gata 3 is essential for luminal cell differentiation during different developmental stages
- gata-3f/f: tree can only grow a bit, no milk producing cells during pregnancy
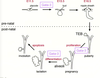
What happens with a loss of gata-3 re:mammary tumours?
- more aggressive
- remove a single allele of gata 3 you get a huge decrease in latency → much more aggressive, came up faster

What does gata-3 promote in tumour cells?
- gata-3 promotes the differentiation of tumour cells and thereby leads to better patient prognosis
- expressed in the stem cell and important for its proliferation
- most important role is in the differentiation of a luminal progenitor to a mature luminal cell

How does breast tumorigenesis occur?
- normal → hyperplasia → atypical hyperplasia → carcinoma in situ (DCIS, LCIS) → invasive carcinoma → metastatic disease
- genetic and epigenetic alterations
- when they break through the basement membrane you have metastatic disease

What is heterogeneity within individual solid tumours?
- e.g. oestrogen receptor expression
- 20 different and very distinct pathological subtypes
- at a molecular level about 6 discreet molecular substypes
- any patient with 1% of cells expressing ER is denoted as ER positive

What are two key questions in breast cancer?
- which cell is the target of transformation in breast cancer - does it result in different tumour subtypes?
- which cells sustain the tumour i.e. can we identify tumour-propagating cells?
Are the ‘cell of origin’ and cancer stem cell the same thing?
- no they refer to different concepts
- cell of origin = cell that experienced first oncogenic hit
- could be any cell along the hierarchy
- CSC = specific subset of cells within the tumour that have the ability to seropassage the tumour
What have gene profiling studies done?
- altered the clinical landscape in breast cancer
- 5 primary molecular subtypes (probably a few more)
- luminal A (ER++)
- luminal B (ER+)
- basal-like
- Erb2/HER2-positive
- claudin low - metaplastic with high expression of proteins like SNAI2, EMT genes
What is true of the four mammary gland subpopulations?
- they have distinct gene signatures
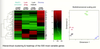
Do different epithelial cells give rise to same subtypes of breast cancer?
- possibly not
- stem cell → claudin low
- luminal progenitor → basal (surprising)
- something on the pathway from luminal progenitor to ductal cell → luminal B or HER2
- ductal cell → luminal A
Are stem or progenitor populations altered in breast cancer prone BRCA1 mutation carriers?
- breast cancer 65%, often early onset
- ovarian cancer 40%
- BRCA1-mutant luminal progenitors exhibit factor-independent growth
- MaSC did not
- highly proliferative /aberrant
- luminal progenitors are the cell of origin for basal breast cancers arising in individuals with BRCA1 mutation s
What can lineage tracing be used for re: tumours?
- lineage tracing can be used to track tumour-initiating cells in vivo
- activate cre-ERT2 in single cell at base of a small intestinal crypt
- track tagged cell in vivo
- e.g. deletion of Apc in stem but not TA cells gives rise to tumours

What is the current status/perspectives on cancer stem cells?
- definition: refers to a subset of tumour cells that can self-renew and generate the diverse cells that comprise the tumour. CSCs can initiate and sustain tumorigenesis
- lie at the apex of the cancer cell hierarchy
- distinct from the ‘cell of origin’
- CSCs do not necessarily originate from the transformation of normal stem cells
- their existence is best demonstrated through serial transplantation of subpopulations into the relevant site
- drugs that kill tumour cells but not cancer stem cells → tumour shrinks but grows back
- they self-renew, multi-potential, relatively quiescent, long-lived
- clonal evolution and cancer stem cell models are contradictory
What is prospective isolation of CSC-enriched populations from solid tumours based on transplantation of sorted cells?
- none of these markers are exclusively expressed by solid tumour CSCs
- even for breast cancer CD44/ALDH1 do not characterise CSCs in every breast cancer
- even of the same type
- difficult area to move in at the moment
- cancer stem cells shift their markers and undergo clonal evolution themselves → not genetically, only epigenetically

What has been identified in mouse models of mammary tumourigenesis?
- identification of ‘cells of origin’ in preneoplastic tissue
- in Wnt1 model increased number of MaSCs but not MMTV-neu mice

What defines CSCs in MMTV-wnt-1 mammary tumours?
- CD61
- CD61+ cells are 20-fold enriched for CSCs

What are cancer stem cell markers in human tumours?
- none of the markers used to isolate CSCs from various cancerous tissues are expressed exclusively by stem cells
- some markers are common between CSCs from different tumours:
- CSC phenotype will not necessarily be uniform between cancer subtypes or even tumours of the same subtype
- what are the similarities between normal stem cells and CSCs? self-renewal pathways


