Lecture 2: the molecules and mechanisms of life Flashcards
(24 cards)
What is a hydroxyl group?
OH functional group
What is an oxonium ion?
RH2O+
(compared to the hydronium ion H3O+)
Distinguish between a carboxylic acid and carboxylate
carboxylic acid is a carbonyl with OH, whereas carboxylate is a carbonyl that has lost its H+ from the OH, so it’s just O-
Distinguish between a carboxylic acid and carboxylic ester
carb. acid = OH functional group on carbonyl
carb. ester = OR functional group on carbonyl
What is the molecular formula for acetic acid, acetate, and an acetyl group?
acetic acid: CH3COOH
acetate: CH3COO-
acetyl group COCH3 carbonyl functional group
What is an acid anhydride?
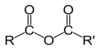
Distinguish between ammonia and the ammonium ion
ammonia = NH3
ammonium ion = NH4+
Distinguish between primary, secondary, and tertiary amines
primary = RNH2
secondary = R2NH
tertiary = R3N
What is an alkyl ammonium ion and a quarternary ammonium ion?
alkyl ammonium ion = RNH3+
quarternary ammonium ion = R4N+
Distinguish between imine and enamine

Distinguish between a thiol and thiolate, sulfide and disulfide, and a thioester
thiol = SH functional group
thiolate lost the H+ from the SH and therefore is a S- functional group
Sulfide = aka thioether R-S-R
Disulfide = R-S-S-R
thioester = carbonyl with an S-R functional group
What is phosphoric acid?
H3PO4

What is inorganic phosphate
HPO42-
also commonly written as Pi

Phosphoric acid is a ____protic acid
tri
What is a phosphoryl group vs a phosphate group?
phosphoryl group is the PO3 functional group attached to a molecule
a phosphate group includes the 4th O and is, therefore, the PO4 group attached to the molecule
What is a phosphoanhydride and pyrophosphate?
Phosphoanhydride refers to 2 phosphoryl groups bonded to each other, in which is then bonded to an R group of some sort: RPO4PO3
Pyrophosphate (aka PPi) are 2 phosphoryl groups bonded to each other: PO4PO3
What are the covalent linkages called that bond each of the following monomers to make macromolecules:
1) amino acids bonded into proteins
2) nucleotides bonded into DNA/RNA
3) monosaccharides bonded into carbohydrates
1) AA are bonded into proteins via peptide linkages
2) nucleotides are bonded into DNA/RNA via phosphodiester bonds
3) monosaccharides are bonded into carbohydrates via glycosidic bonds
The intermediary between the info stored in DNA and the info manifested in proteins is _____, synthesized using DNA as a template and acting as the tempalte for protein synthesis
mRNA
The “native folding” of a protein depends on _______, which then determines the function of the protein
amino acid sequence
Distinguish between nucleophiles and electrophiles
nucleophiles are nucleus-loving and, thus, are e- rich and seek to bond with molecules/atoms with a (partial) positive charge
electrophiles are electorn-loving (aka e- deficient) and, thus, are attracted to molecules/atoms with a negative charge or that are e- rich
Distinguish between SN1 and SN2 rxns
SN1 generates a carbocation intermediate and, therefore, proceed in a 2-step mechanism. The LG leaves, creating a carbocation (which may be prone to carbocation rearrangement if it produces a more stable cation), and then the Nu attacks the carbocation
SN2 proceeds in a 1-step mechanism and, therefore, generates no intermediates. The Nu attacks the Electrophile at the same time the LG breaks its bond with the electrophile
Distinguish between E1 and E2 rxns
E1 rxn creates a carbocation or carbanion intermediate
E2 does not create a carbocation intermediate and, therefore, is a concerted rxn
Both create a C=C, but most rxn mechanisms are prone to the E2 rxn mechanism instead of an E1 rxn mechanism. The dehydration and rehydration of molecules will be the most common elimination rxn seen in biochem
alcohols and carbonyls will react together in a nucleophilic carbonyl addition rxn to generate a ____ or _____
hemiacetal or acetal
this type of rxn occurs frequently in carbohydrates; there is a tetrahedral intermediate with this rxn mechanism


