Lecture 14 Haemostasis and Thrombostasis Flashcards
What are the sub-classifications of bleeding
Primary and secondary haemostasis
What are the sub-classifications of clotting
Arterial and venous thrombosis
What three fractions are isolated from fresh whole blood following donation
Red blood cells platelets and plasma
How long can red blood cells be kept and used for after donation
35 days
How long can platelets be kept and used for after donation
5-7 days
How long can plasma be kept and used for after donation
Can be frozen and stored for quite some time before use
How can fresh frozen plasma be further divided
Plasma can be separated into the individual clotting factors
What is the first response to bleeding
Localised vasoconstriction
What are the two components to the clotting system
Primary haemostasis – platelet activation and adhesion. Secondary haemostasis – activation of the coagulation cascade
What is the role of the coagulation cascade
To produce fibrin that fixes the cells together
What are the two causes of platelet bleeding disorders
Reduced platelet numbers (thrombocytopenia) or impaired platelet function that is either inherited or acquired
How are patients with platelet bleeding disorders treated
Platelet transfusions
How to platelets bind to collagen in the endothelium during clotting
Via the binding of von Willebrand factor
What is the inheritance pattern of von Willebrand disease
Autosomal dominant
Von Willebrand disease is the most common inherited bleeding disorder with as many as 10% of the population having reduced vWF levels T or F
F – whilst it is the most common inherited bleeding disorder its 1% of the population who have reduced vWF levels
Von Willebrand disease is a milder bleeding disorder to haemophilia T or F
T
What are the main symptoms of von Willebrand disease
Bruising cuts mennohagia epistaxis (nose bleeds) and bleeding of the gums
What are the two approaches in the treatment of von Willebrand disease
Desmopressin to stimulate endothelial cell degranulation of the Weibel-Palade bodies that contain vWF. Alternatively intermediate purity/plasma-derived factor VIII is given which contains vWF
What is the name of the granules that contain vWF in the endothelial cells
Weibel-Palade bodies
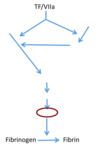
What factor is activated first in the imitation of secondary haemostasis
Tissue factor or clotting factor VIIa
What is the main activator of the coagulation cascade in humans
Clotting factor VIIa
How is factor VIIa activated during vessel injury
Injury to the vessel exposes FVIIa which is underneath the vessel
What are the two effects of activating factor VIIa
FVIIa both directly and indirectly leads to the conversion of factor X to factor Xa. It directly stimulates this conversion but also acts via triggering the conversion of factor IX to IXa which then stimulates factor X conversion
Which pathway of factor Xa production is more efficient
The indirect pathway