Lecture 10- Introduction to metabotropic receptors Flashcards
What are the two types of synaptic transmission?
- Ionotropic neurotransmission
- Metabotropic neurotransmission
What are the general characteristics of ionotropic neurotransmission?
- fast, never uses peptide neurotransmitters
- small molecules used (dopamine etc.) and recycled within the terminal
- leads to and ESPS (Na+ inflow ) or ISPS (Cl- inflow)
What are the general characteristics of metabotropic transmission?
- does not cause ESPS/ISPIS
- yet usually results in a change in ion coductance and membrane potential
- metabotropic receptors are G-protein coupled receptors
- modulate the transmission between neurons
What are the general characteristics of metabotropic transmission?
- does not cause ESPS/ISPIS
- yet usually results in a change in ion coductance and membrane potential
- metabotropic receptors are G-protein coupled receptors
- modulate the transmission between neurons
- makes the secondary neuron more or less receptive
What do G-proteins do?
-they are adapters, they link receptors to effectors
What do the G-protein coupled receptors look like?
- have 7 transmembrane domains
- have a ligand binding region in the extracellular facing region
- various bits poke out of the cytoplasm that are responsible for binding the G proteins
- G-protein coupled receptors are useless alone, but can activate a G-protein that can then do things
- the G-protein coupled receptors are referred to as serpentine due to the 7 TM domains
- have no intrinsic activity

What are the 3 components involved in a G-coupled protein receptor exerting its effect on the system?
1: G-protein coupled receptor
2: G-protein
3: Effector proteins (usually ion channels and enzymes that generate 2nd messengers)
What are the 3 components involved in a G-coupled protein receptor exerting its effect on the system?
1: G-protein coupled receptor
2: G-protein
3: Effector proteins (usually ion channels and enzymes that generate 2nd messengers)
- the GPCRs use G-proteins to couple to effector proteins
What is the diversity of G-proteins?
– There are 20 different α subunits
– There are 5 different β subunits
– There are 12 different γ subunits – Thus 1200 possible G-proteins
- most of which actually occur!
- alpha, beta and gamma subunits of the G protein
- alpha splits from beta and gamma readily (beta and gamma never split)
- alpha activates the effector -extraordinary diversity of the G proteins
- they will activate different things -mix and match system
What is the effect of metabotropic stimulation?
- Metabotropic stimulation often alters ion-channel permeability
- The change in permeability is slower in onset, and of longer duration, than occurs with ionotrophic stimulation
What are the 3 ways in which metabotropic receptors regulate ion channels?
- Coupling by G-protein directly to an ion channel
- Coupling by G-protein to a second messenger system, where the 2n messenger (eg. cAMP, cGMP) directly regulates an ion channel
- Coupling by G-protein to a second messenger system, leading to ion channel phosphorylation
- proteins that are usually phosphorylated are ion channels so the membrane permeability is different
- long-winded way of doing it
What does the release of second messengers do?
• Ultimately, release of second messengers results in phosphorylation of specific subsets of cellular proteins
What are the phosphorylation targets of the second messengers?
• Phosphorylation targets include:
– Ion channels
– Ion pumps
– Receptors
– Enzymes
– Structural proteins (to lesser extent enzymes and structural proteins
-mostly the first three and they are all transmembrane structures so can affect membrane permeability)
What is a signalosome?
- Any of a group of proteins, complexes of which are involved in the regulation of protein degradation
- arranged in a way that they can easily interact, not just randomly spread out around the membrane
- this is how the GCPRs, G-proteins and effectors are arranged
Are all three components of the G-coupled protein receptor machinery membrane bound?
-yes -the GCPRs, the G-protein and the effector
How are GCPRs coupled to effector proteins?
-use the G-proteins to have an effect on the effector
What are the main effector proteins?
-ion channels and enzymes that generate 2nd messengers
What happens when a signalling molecule binds to a GCPR?
- G protein has a GDP bound to its alpha subunit in its resting state
- once the signaling molecule attaches to the GCPR the GDP is converted to GTP and this activates the alpha subunit and causes a split of the alpha subunit from the beta+gamma subunits (these stay together)
- the alpha subunit will then go on to activate the effector
- as long as the alpha subunit has the GTP it remains active, but GTP is unstable and breaks down in about 20 seconds (too long!) so have GAPs (G protein associated proteins
What does the beta+gamma subunits do when a signaling molecule binds to the GCPR?
- it is not only the alpha subunit that does something often the beta and gamma are also active
- sometimes perform similar functions to the alpha subunit and sometimes do the opposite: eg. G protein where alpha subunit excites adenylyl cyclase, the beta and gamma subunits inhibit it but take longer to have an effect so it is a sort of built in off switch
What happens when a signaling molecule binds to a GCPR?
- G protein has a GDP bound to its alpha subunit in its resting state
- once the signaling molecule attaches to the GCPR the GDP is converted to GTP and this activates the alpha subunit and causes a split of the alpha subunit from the beta+gamma subunits (these stay together)
- the alpha subunit will then go on to activate the effector
- as long as the alpha subunit has the GTP it remains active, but GTP is unstable and hydrolyzes into GDP in about 20 seconds (too long!) so have GAPs (G protein associated proteins) whose job is to speed up the GTP hydrolization so it only takes milliseconds
- once the GTP is converted to GDP everything stops and alpha dissociates from the effector and associates with beta and gamma at the GCPR again
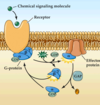
What does the beta+gamma subunits do when a signaling molecule binds to the GCPR?
- it is not only the alpha subunit that does something often the beta and gamma are also active
- sometimes perform similar functions to the alpha subunit and sometimes do the opposite:
eg. G protein where alpha subunit excites adenylyl cyclase, the beta and gamma subunits inhibit it but take longer to have an effect so it is a sort of built in off switch
What is the diversity of G protein coupled receptors and G proteins?
-there are >1000 G-Protein Coupled Receptors G-proteins also exhibit enormous diversity
– There are 20 different α subunits
– There are 5 different β subunits
– There are 12 different γ subunits
– Thus 1200 possible G-proteins
- most of which actually occur!
What was the initial classification of the G proteins like?
-according to effect of alpha subunit
- Gαs stimulates Adenylyl Cyclase
- Gαi inhibits Adenylyl Cyclase (sensitive to pertussis toxin= whooping cough acts here)
- Gαq stimulates Phospholipase C
- Gαt Inhibits cGMP Phosphodiesterase (cGMP Phosphodiesterase breaks down cGMP)
Can the beta-gamma complex be an effector and if then why are there so many different effector possibilities?
-yes
• We now have 20 + 60 = 80 different effector possibilities…. So why are there 1200 different G-proteins?
The answer probably is in the existence of signalsomes: pre- formed scaffoldings in which GPCR, G-protein, effector and regulatory molecules are locked into position
- 80 different effectors so can only have 80 effects
- have so many G proteins since can for a G protein have an effect in one cell and another effect in another cell
- also since they exist in signosomes, not random along the membrane, they are preformed with scaffolding proteins holding things together
What are these?
- some of the common effectors
- some G proteins target cAMP phosphodiesterase (breaks down cGAMP)
- some target cGMP phosphodiesterase (breaks down cGMP)
- the cAMP and cGMP are important signaling molecules
- G proteins can modulate the system in lot of different ways
What is this?
- do not have to memorise this
- showing how the G proteins affect the system and thus the electrochemical properties of the cell

At which stage of the system is there amplification possibility?
- from receptor to G proteins
- from adenylyl cyclase to cAMP
- from protein kinases to phosphates ( as long as it is active it will phosphorylate)
- lot of possibility for this system to amplify and get out of control!
What happens when you get long-term G protein effect?
-will get effects in the nucleus and will have some transcriptional effect (CREB for example), then much more effect
How are the receptor signals switched off?
- detachment of ligand (ligand affinity lasts a short time)
- hydrolysis of GTP
- removal of 2nd messengers (may be labile, removed or destroyed
- activation of phosphorylases
- if the hydrolysis doesn’t happen get overactivation of G proteins and tendency to have tumours (people with lot of tumours on their skin have this condition neurofibriloma)
How are proteins affected by kinases and phosphotases?
- cellular proteins are regulated via phosphorylation
- have protein that is phosphorylated via protein kinase (that is switched on via second messengers)
- the protein becomes phosphoprotein
- the phosphoprotein is returned to its normal state via protein phosphatase that removes the phosphate (also acted on via second messengers)
- this process is crucial for the system to be a signal, must be transient, otherwise not a signal
How are proteins affected by kinases and phosphotases?
- cellular proteins are regulated via phosphorylation
- have protein that is phosphorylated via protein kinase (that is switched on via second messengers) -the protein becomes phosphoprotein
- the phosphoprotein is returned to its normal state via protein phosphatase that removes the phosphate (also acted on via second messengers)
- this process is crucial for the system to be a signal, must be transient, otherwise not a signal
What are the characteristics of peptide neurotransmitters?
- Peptide neurotransmitter receptors are all metabotropic
- Peptide neurotransmitters are synthesized and packaged into vesicles in the cell body
- They are then transported along microtubules to the axon terminals
- They undergo exocytosis in response to extensive calcium influx
- dopamine etc aree small molecules= synthesised at the terminal but peptides are not!
- peptides: transported along microtubules -peptides released slowly, not to a quick spike to calcium, must be a long spike
What are some examples of important peptide neurotransmitters?
- Substance P (pain transmission)
- Neuropeptide Y (blood pressure, appetite stimulation)
- Angiotensin II (thirst and salt appetite)
- Endorphins (pleasure, reward, inhibition of pain)
What does the system with peptide neurotransmitters work like?
- Unlike small-molecule vesicles, peptide vesicles are not recycled at the nerve terminal
- The fused vesicular membrane is endocytosed and transported back to the cell body
- There is no re-uptake of the peptide
- Peptide transmitters undergo proteolytic cleavage after release
- broken down and only used once
- often the vesicle releasing the peptide contains the proteolytic cleavage things
What are the LDCVs?
- Vesicles containing peptide transmitters are known as Large Dense-Core Vesicles (LDCVs) • They are 200 - 400 nm in diameter (cf 40-50 nm)
- They appear dense under the electron microscope
- In addition to the transmitter, they contain proteins which act as a scaffolding
- In some cases they contain peptidases which may act after release
What is common with the number of neurotransmitter at a terminal?
-often a nerve terminal will have two neurotransmitters= a small molecule one released frequently and peptide rvesicles relased infrequently and modulate the other
What is the general role of metabotropic receptors in synaptic transmission?
- usually modulatory but sometimes can be very quick: retina, photon get in and then G protein
- membrane voltage difference and that is transmitted to the next cell in the line
What is the role of metabotropic receptors in synaptic transmission as neurotransmitters?
they can act as excitatory or inhibitory neurotransmitters that depolarize or hyperpolarize the membrane, respectively. Similar to ionotrophic receptors, but not as fast
What is the role of metabotropic receptors in synaptic transmission as neuromodulators?
they alter the excitability of the post-synaptic neuron, but don’t regulate the membrane voltage directly
What is the role of metabotropic receptors in synaptic transmission as co-transmitters?
An example of neuromodulatory action, but released from the same synapse as a fast neurotransmitter
What is the role of metabotropic receptors in synaptic transmission as autoreceptors?
Present on the pre-synaptic terminal, they inhibit release of neurotransmitter
What is the role of metabotropic receptors in synaptic transmission as non-synaptic receptors?
An example of neuromodulatory action, but located outside of the synapse altogether.
What are co-transmitters?
- Synapses often contain two types of vesicle: a small-molecule vesicle (usually glutamate), and a peptide-containing vesicle
- These may be co-released
- Release of LDCVs requires more prolonged stimulation
- Co-transmitters are modulatory: sometimes synergising with, and sometimes opposing, the ionotropic effect
What are autoreceptors?
- Presynaptic
- Inhibit VACC
- Inhibit release of neurotransmitter
- negative feedback receptors, first discovered at dopaminergic terminals
- had dopamine receptors on the dopaminergic terminal itself (the autoreceptor
- if the amount of dopamine in the synaptic cleft too high then this discourages further dopamine release, this also exists serotonin, adrenaline etc… always a negative feedback function
- prevent over-release, they are g protein coupled, work by altering calcium (reducing the levels inside teh termina) and reducing stimulus for release via that

How do metabotropic synapses have a different morphology?
- Whereas ionotrophic receptors are clustered tightly within the PSD, metabotrophic receptors are more spread out.
- Their PSDs are less dense - some receptors lie outside the PSD
- In some cases, synapses as such do not exist: eg release of transmitters from autonomic nerves occurs in close proximity to the smooth muscle it innervates.
- The receptors - which are all metabotrophic - are distributed over the surface of the smooth muscle
- not quite a synapse sometime, sometimes spread out (PSD= postsynaptic density)
How does desensitisation occur?
- if the GCPR system is stimulated for too long it will desensitise
- this happens as some of the kinases activated by the G protein some phosphorylate the receptor itself
- the more phosphate groups are added the more likely that GRK and Arrestin will be added and these cause internalisation of the G protein coupled receptor
- even before phosphorylation means less affinity to binding so less active
What is the function of Phospholipase C?
- it is one of the most important targets of the GCPR and it targets PIP2
- PIP2 is through Phospholipase C split into two active molecules IP3 and diacylglycerol
- IP3 has 3 phosphates, intracellular molecule and has its own receptors and affects the intracellular calcium stores, leads to release of calcium
- digacyglycerol is in the membrane and activate protein kinase C
What sort of transmitters are metabotropic neurotransmitters?
Transmitters such as the catecholamines and peptides are metabotropic transmitters
Can ionotropic neurotransmitters also be metabotropic neurotransmitters?
Ionotropic neurotransmitters can also be metabotropes
– muscarinic ACh receptor
– metabotropic Glutamate receptor (mGluR)
– GABAB receptor
- glutamate we think of as ionotropic but also have metabotropic receptors
- even serotonin is, one receptor of the 20 is metabotropic
- peptides have only metabotropic though!
What determines the nature of the transmission? Transmitter/receptor
it is the receptor, not the transmitter, that determines the nature of neurotransmission


