Lab 3: Spirometry Flashcards
Respiration definition:
•The exchange of oxygen and carbon dioxide across membranes (either lungs or cellular level)
Ventilation Definition:
•Movement of air into and out of the lungs
Diffusion Deffinition:
•Movement of gases between the alveoli and blood along a concentration gradient
Perfusion Defiinition:
•The passage of blood through the blood vessels to tissues
Air conducting portion:
Nasal cavities, Nasopharynx, Larynx, Trachea, Bronchi, Bronchioles and terminal bronchioles.
Provides a conduit through which air moves to and from the lungs.
Conditions the inspired air.
Air respiratory portion
Respiratory bronchioles, Alveolar ducts, Alveoli.
Function is gas exchange.
Functions of dead space:
- Warms and humiditifies air
- traps and remoces inhailed particals by mucocilliary transport
- Retains CO2 by mucocilliary transport
- Phonation
Factors that affect anatomical dead space and alveolar dead space
Factors that affect anatomical dead space:
- Body size, age, lung volume – increase anatomical dead space
- Supine position (lying flat on your back) compared to sitting – decreases anatomical dead space
- Hypoxia – decreases anatomical dead space due to bronchoconstriction
Factors that affect alveolar dead space:
- Age increases alveolar dead space
- Decreased pulmonary arterial pressure -> decreased in perfusion to upper part of the lung (zone1) -> increases alveolar dead space
Nitrogen Washout (Fowler’s) Method
- A single-breath nitrogen washout test, used to calculate anatomical dead space (and closing capacity)
Meathod:
- At the end of a normal tidal breath (at FRC) a vital-capacity breath of 100% oxygen is taken
- The patient then exhales to RV
- Expired nitrogen concentration and volume is measured.
- A plot of expired nitrogen concentration by volume is generated
- Phase 1 (Pure Dead Space): Gas from the anatomical dead space is expired. This contains 100% oxygen - no nitrogen is present.
- Phase 2: A mix of anatomical dead space and alveolar (lung units with short time constants) is expired. The midpoint of phase 2 (when area A = area B) is the volume of the anatomical dead space.
- Phase 3: Expired nitrogen reaches a plateau as just alveolar gas is exhaled (lung units with variable time constants).
Example.
- Out of 500ml inspired air, only 350ml reaches alveoli for gas exchanges.
- The rest 150mL just fills the anatomical dead space.
- So, during expiration, first 150ml = dead space and last 350ml = alveolar air.

Physiological dead space

Dead space definition:
The proportion of minute ventilation which does not participate in gas exchange.
Anatomical:
- in conducting airways
- volume of air in the parts of the aiways not involved in gas exhange
- ~150 ml
- “series dead space”
Alveolar:
- amount of inspired air not used for gas exchange with the blood
- this is due to ventilation/perfusion mismatch
- “parallel dead space”
Alveolar Ventilation definition
•Rate at which air reaches the alveoli
Minute Ventilation =
Minute ventilation (VE): volume of air that a person breathes per minute (i.e., the product of tidal volume and respiratory rate; VE = VT x RR)
Total volume of gas entering the lungs per minute
- Minute Ventilation = Respiratory rate x Tidal volume
- ~250 ml O2/min
Alveolar ventilation
- The volume of gas per unit time that reachs the alveoli ( minus the dead space)
- Alveolar ventilation = Respiratory rate x (Tidal volume – volume of dead space)
AV = RR x (TV – dead space)
∴ AV = 12 x (0.5 – 0.15)
∴ AV = ∼4.2L/min
Minute Ventilation and Exercise
- Volume increases from 0.5 to 4.6 liters
- Bpm increased from 12 to 24 breaths/min
Minute Ventilation = Respiratory rate x Tidal volume
- The minute vent. has therefore increased from 6.0 L to 120 L/min i.e. more than a twenty-fold increase!
stav: from 0.5 to 2.8 liter
bpm is same
minute ventilation: from 6 liters to 64.4 l/min: 10 times more
Functions of the Respiratory System
PH: After we know all about the dead space, what would be the result of breathing too fast and shallow?
Loss of CO2, therefore making the blood more basic (increased pH)

Fick’s law of diffusion
????????
- Fick’s law measures the gas exchange by simple diffusion through cell membranes or capillary walls.
- It states that the rate of diffusion across a membrane is
- directly proportional to the concentration gradient of the substance on the two sides of the membrane
- inversely related to the thickness of the membrane.
Composition of air
- In atmosphere
*
- 21% oxygen
- less than 1% carbon dioxide etc.
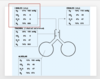
Composition of air in trachea:
When air enters respiratory center it is vaporized/humidified therefore drop in Po2 as no longer 21% because H2O % is added

Compotition of air in alevoli/ Driving force for diffusion
What is the driving force for diffusion?
- In the interstitial fluid the partial pressure of O2 is around 40 mmHg = blood O2 in capillaries at the venous end
- This difference between the pressure in the blood entering the pulmonary capillaries and that leaving the lung itself creates a pressure gradient which is the driving force for diffusion i.e. Fick’s Law

Gas exchange at alveolar and systemic capillaries: draw scheme
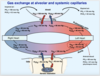
What are the effects of ventilation on blood gases?
Hyperventilation
- Affects CO2 release more than O2 uptake
Hypoventilation
- Minor: affect CO2 release more than O2 uptake
- Major: affect O2 uptake more than CO2 release
Why is there is a slight decrease in the partial pressure of oxygen from the alveoli into the blood (100mmHg à95mmHg)?
- Because 98% of the blood is from pulmonary capillaries that has a po2 of 100mmhg
- BUT there is also 2% coming from the bronchial vein that has a po2 of 40mmhg
- This is called a physiological shunt i.e. a venous admixture of bronchial vein with deoxygenated blood draining into the pulmonary vein carrying oxygenated blood to the left heart.

How much O2 is delivered to the tissues per minute?
- 1 gram of haemoglobin can carry 1.39 ml O2 at 100% saturation, normally less 1.34 ml (decreased purity)
- We have 15g Hb/100ml of blood
- Therefore, 20.1ml O2 in 15g Hb per 100ml of blood (15 x 1.34 = 20.1)
***This bound form does not contribute to Po2
- Therefore, as cardiac output is 5l/min, we can say there is 1L O2 in total blood (20.1 x 10 x 5 = 1000ml)
- Therefore at 75% saturation then 0.75*1L = 250ml o2/min is delivered to tissues per min
- 200ml CO2 produced per min, this is less than the 250ml O2 because H2O is also produced.
Why is the difference in partial pressure of carbon dioxide much less than that of oxygen?
*********
*200ml CO2 produced per min, this is less than the 250ml O2 because H2O is also produced.
Chloride shift:
- refers to the transporter protein on the RBC membrane which transports chloride ions into the cell and HCO3- out, maintaining neutrality of charges.
Excess intracellular HCO3− produced this way is released into the plasma in exchange for Cl-.
Bicarbonate buffer system
- RBCs carry carbonic anhydrase,
- which converts HCO3- and H+ to H2O and CO2 in the following steps:
- HCO3− + H+ ⇄ H2CO3 ⇄ H2O + CO2
- Ultimately, excess H+ during acidic states is eliminated through conversion to CO2, which can be exhaled.
- During basic states, the bicarbonate buffer system can reverse so that CO2 is converted to HCO3− + H+.
Chloride shift: Excess intracellular HCO3− produced this way is released into the plasma in exchange for Cl-.
- This phenomenon makes HCO3- the most important buffer in the body.
- HCO3- accounts for 50% of the blood buffer capacity.
- For more details on the buffering mechanisms of the body, see compensation in acid-base disorders.
Carbon dioxide and blood pH
Make sure you understand how the blood pH can change due to changes in concentration of carbon dioxide. It is essential you understand this in order to understand the next slide; Bohr effect.
Higher H+ = Acidic, low pH
CO2 is mainly carried in three forms in the body:
- Plasma bicarbonate, HCO3- (70%)
- Bound to Hb (carbamino-Hb, HbCO2) at the N-terminus of globin (does not compete with O2 at heme) (23%)
- Dissolved in blood: Plasma (7% - gives pCO2 level)
- PaCO2 = 40mmHg
- PvCO2 = 46mmHg
2 main types of hemoglobin:
Oxygenation and deoxygenation of Hb
- Oxyhemoglobin
- Hemoglobin with oxygen bound to its heme component (oxygenated) → bright red blood
- Oxygenated hemoglobin has approximately 300 times higher affinity for oxygen compared to deoxygenated hemoglobin
- Exhibits positive cooperativity and positive allostery
- IR
- Deoxyhemoglobin
- Hemoglobin with no oxygen bound to its heme component (deoxygenated) → dark red blood
- Blue to purple appearance of tissue during hypoxia → “cyanosis”
- Deoxygenated hemoglobin has a low affinity for oxygen → release of oxygen is promoted
- RED LIGHT
Oxygen Transport: Transported as
- OxyHb (97%)
- Plasma (3% - gives pO2)
- PAO2 = 100-110mmHg
- PaO2 in pulmonary circulation = 100mmHg
- PaO2 in systemic circulation = 95mmHg
- PvO2 = 40mmHg
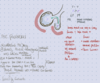
Bohr effect
- The O2 affinity of Hb is inversely proportional to the CO2 content and H+ concentration of blood.
- High CO2 and H+ concentrations (from tissue metabolism) cause decreased affinity for O2. → O2 that is bound to Hb is released to tissue (the O2-Hb dissociation curve is shifted to the right).
- HbO2 + H+ ⇄ H+Hb + O2
- HbO2 + CO2 ⇄ Hb-COO- + H+ + O2
•Increase in blood carbon dioxide →decrease in pH → unloading of oxygen.

Haldane effect
- The CO2 affinity of Hb is inversely proportional to the oxygenation of Hb.
- When Hb is deoxygenated (typically in peripheral tissue), uptake of CO2 is facilitated.
- When Hb is oxygenated (in high pO2, for example, in the lungs):
- Oxygenated Hb has a decreased affinity for CO2. → CO2 that is bound to Hb is released in the pulmonary arteries to diffuse into the alveoli (the O2-Hb dissociation curve is shifted to the left).
- Hb releases bound H+ → ↑ H+ shifts equilibrium to CO2 production (see equation above) → CO2 is exhaled in lungs
Haldane Effect: Oxygenation of blood in the lungs displaces carbon dioxide from hemoglobin which increases the removal of carbon dioxide. Consequently, oxygenated blood has a reduced affinity for carbon dioxide. Thus, the Haldane effect describes the ability of hemoglobin to carry increased amounts of carbon dioxide (CO2) in the deoxygenated state as opposed to the oxygenated state. A high concentration of CO2 facilitates dissociation of oxyhaemoglobin. Only an “empty” Hb likes CO2
Regulation of respiration:
- The basic regulatory rhythm is generated in dorsal medulla
- Respiratory control is autonomous, chemical, and neuronal.

What are the different types of inspiration and what is involved?
- Normal/quiet breathing = diaphragm
- Forced breathing = diaphragm + external intercostals
- Laboured breathing = diaphragm + external intercostals + accessory muscles
Carbon- hemoglobin dissosiation curve

What are the different types of expiration and what is involved?
- Passive – no muscle contractions (relaxation of inspiratory muscles and elastic recoil of lungs)
- Forced - muscles
Inspirationa and expiration: Principle and acessory muscles

Inspiration: mechanism

Expiration mechanism

Lung Volumes and Capacities: General overview
Total lung capacity (TC,TLC)
Volume of air in the lungs after maximal inhalation TC = VC + RV
6–6.5 L
Vital capacity (VC)
Difference in lung volume between maximal exhalation and maximal inhalation (VC = TV + IRV + ERV)
*The vital capacity is the volume of air in the lungs that is available for gaseous exchange.
4.5–5 L
Residual volume (RV)
Volume of air that remains in the lungs after maximal exhalation
1–1.5 L
Tidal volume (TV)
Volume of air that is inhaled and exhaled in a normal breath at rest
∼ 500 mL or 7 mL/kg
Inspiratory reserve volume (IRV)
Maximum volume of air that can still be forcibly inhaled following the inhalation of a normal TV
3–3.5 L
Inspiratory capacity (IC)
Maximum volume of air that can be inhaled after the exhalation of a normal TV (IRC = IRV + TV)
3.5–4 L
Expiratory reserve volume (ERV)
Maximum volume of air that can still be forcibly exhaled after the exhalation of a normal TV
1.5 L
Expiratory capacity (EC)
Maximum volume of air that can be exhaled after the inspiration of a normal TV
ERC = ERV + TV
2 L
Functional residual capacity (FRC)
Volume of air that remains in the lungs after the exhalation of a normal TV (FRC = RV + ERV)
2.5–3 L
Normal and pathologic ventilation

Factors that affect lung volume

What are thr pressures within lung?

What is the transpulmonary pressue value?
Alveolar Pressure:

Ventilation-perfusion mismatch (V/Q mismatch)
Lung compliance
- Definition:
- The ability of the lungs to distend under pressure
- Inversely proportional to elastance, which is resistance to distention created by elastic properties of the lung tissue
- Measurement:
- Change in volume of the lung per unit change in pressure (C = ΔV/ΔP)
- ΔP= Transpulmonary Pressure
= Intraalveolar Pressure - Intrapleural Pressure
- Increased in:
- Emphysema (lungs fill easier), aging
- Decreased in:
- Conditions associated with increased lung stiffness (e.g., pulmonary fibrosis, pulmonary edema, pneumoconioses, ARDS)
*Surfactant reduces the surface tension in the alveoli and thus increases the compliance of the lungs.
*In old age, lung compliance increases due to loss of elastic recoil, while chest wall compliance decreases because the chest wall stiffens.

Transpulmonary Pressure could it be 0?
could be 0mmHg when coughing or sneezing
Elastance
- Definition = ability of a substance to recoil
- Opposing force of compliance
- Elastance = ∆P/∆V
- Formed from:
–Elastin
Chest wall dynamics:
-
Resting expiratory position (functional residual capacity)
- Thorax pulls outward and lungs pull inward (due to the passive elastic recoil of the lungs)
- Alveolar and airway pressure = atmospheric pressure (state zero)
- Atmospheric pressure (zero state) = point of functional residual capacity
- When FRC is at zero, the pulmonary vascular resistance is also at its lowest
- Intrapleural pressure is negative (to keep lungs expanded and prevent atelectasis)
-
Inspiration
- Intrathoracic pressure becomes even more negative to fill the lungs with air
- Inspiratory muscles: mainly external intercostal muscles and the diaphragm (elevate ribs and sternum)
- ***During inspiration (in resting conditions) the pleural pressure moves from -5 cmH2O to
- 7.5 cmH2O. See the graph.
-
Expiration
- Intrathoracic pressure becomes positive to expel the air (passive elastic recoil of the lungs)
- Expiratory muscles: mainly internal intercostal muscles, innermost intercostalis muscles, and subcostal muscles
Lung- Thorax relatation pressure curve

Lung Volumes during changes in transpulmonary pressure
???
Airway resistance:

What produces surfactant, where is it stored?
- Surfactant is produced by Type 2 Pneumocytes,
- Stored in Lamellar bodies and moved to the surface by exocytosis
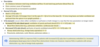
What problems do surface tension create as:
- Tendency of alveoli to collapse,
- Unequal Alveoli
- Tendency of edema
•As shown on the left without surfactant due to the smaller pressure the air will move to the larger bubble and the smaller bubble will deflate and so the smaller alveolus will be under ventilated.
This would cause some of of the blood traveling to the smaller bubble to not be oxygenated i.e. change in ventilation:perfusion ratio.
The high collapsing pressure in the smaller alveolus can cause water to move from capillaries into the alveolus i.e. EDEMA!
•Law of LaPlace:
oCollapsing Pressure (P) = (2 x Surface Tension (T)) / Radius of Alveolus (r)
Surfactant molecule
- Surfactant molecule = 90% phospholipid + 10% protein (Apoprotein A, B, C, D)
- Dipalmitayl Phosphotidyl Choline
- Amphipathic (Both hydrophobic and hydrophilic parts)
- Production
- begins at 24th week, enough surfactant by 35th week.
- Therefore, baby born before 35th week, not enough surfactant has been produced causing Respiratory Distress Syndrome.
- Apoproteins A + D act as opsonins to facilitate phagocytosis. IgA also aids in immune function.
- Apoprotein B + C help surfactant to spread over surface

Perfusion
Process of the body delivering blood to a capillary bed in its biological tissue
- V/Q = Ventilation/Perfusion
- V/Q Ratio = Ratio of alveolar ventilation per minute to quantity of blood supplied to alveoli
•Normal V/Q Ratio = 4L/5L = 0.8
•Shunt/ Dead Space
Pulmonary blood flow
Pulmonary circulation:
- the blood flow is equivalent to cardiac output (∼ 5 L/min)
- Distribution of blood flow:
- depends on the position of the body and is precisely regulated in relation to the ventilation to optimize gas exchange
- Standing and sitting position: due to gravity, circulation is highest in the lung base
- Supine position: distribution of the blood is nearly equal throughout the lung
Regulation of pulmonary blood flow
- Ventilation-perfusion ratio:
- the perfusion of the pulmonary circulation can be regulated to match the ventilation of the alveoli in order to optimize gas exchange
- The ventilation-perfusion ratio is higher in the lung tip than in the lung base →
- alveolar O2partial pressures are higher in the lung peak than in the lung base
- If a lung section is perfused but not ventilated, there is a drop in the oxygen concentration in the blood →
- hypoxic vasoconstriction (Euler-Liljestrand mechanism)
- In order to keep the ventilation-perfusion ratio constant, the vessels of the lungs react to hypoxia with vasoconstriction.
- In contrast, hypoxia in other organs causes vasodilation to increase perfusion.
The apical lung segments have higher O2partial pressures because the perfusion in these lung segments is lower than the ventilation and thus less O2 diffuses from the alveoli into the bloodstream. Some microorganisms (e.g, M. tuberculosis) favor apical lung segments due to the higher O2 content.
During exercise, the increased cardiac output from the right ventricle increases pulmonary circulatory pressure, which then opens apical blood vessels that were initially collapsed. This allows for perfusion in that region, thereby reducing dead space (V/Q ratio ≈ 1).
What is Spirometry

Lung volumes and capacitues: values
Lung Volumes:
Inspiratory Reserve Volume: 3100ml
Tidal Volume: 500ml
Expiratory Reserve Volume: 1200ml
Residual Volume: 1200ml
Lung Capacities:
Inspiratory Capacity: 3100ml + 500ml = 3600ml
Functional Residual Capacity: 1200ml + 1200ml = 2400ml
Vital Capacity: 3100ml + 500ml + 1200ml = 4800ml
Total Lung Capacity: 3100ml + 500ml + 1200ml + 1200ml = 6000ml
What are Dynamic Lung Functions?
Lung volumes that depend upon the rate at which air flows out of the lungs are termed dynamic lung volumes.
There are various dynamic tests: Forced Vital Capacity test, and the Maximum Voluntary Ventilation test.
Types of dyanmic lung parameters/functions:

•Forced vital capacity (FVC)
– total volume expired by a forceful maximal expiration from a position of maximal inhalation
*FEV1/FVC gives us a ratio used to determine physiological and pathological states (restrictive vs obstructive lung diseases)
•Forced expiratory volume in 1 sec (FEV1)
– volume expired in first second of maximal forceful expiration from a position of full inhalation
*FEV1/FVC gives us a ratio used to determine physiological and pathological states (restrictive vs obstructive lung diseases)
•Forced expiratory ratio (FER)
in %
–FEV1/FVC = Tiffeneau index
•Peak expiratory flow rate (PEFR)
– highest forced expiratory flow in one breath (L/min)
PEF = Peak Expiratory Flow = maximum speed of expiration that can be achieved in one breath instantaneously
used to evaluate patients lung capacity and monitor asthmatics
•Forced expiratory flow rate
(FEF25-75%) – average flow rate measured over the middle half (50%) of the FVC (related to FEV1)
FEV1/FVC
Tiffeneau index
FEV1/FVC gives us a ratio used to determine physiological and pathological states (restrictive vs obstructive lung diseases)
- It represents the proportion of a person’s vital capacity that they are able to expire in the first second of forced expiration (FEV1) to the full, forced vital capacity (FVC).[4] The result of this ratio is expressed as FEV1%.
- Normal values are approximately 75%.[5] Predicted normal values can be calculated online and depend on age, sex, height, and ethnicity as well as the research study that they are based upon.
- A derived value of FEV1% is FEV1% predicted, which is defined as FEV1% of the patient divided by the average FEV1% in the population for any person of similar age, sex, and body composition.
In obstructive lung disease, the FEV1 is reduced due to an obstruction of air escaping from the lungs. Thus, the FEV1/FVC ratio will be reduced
In restrictive lung disease, the FEV1 and FVC are equally reduced due to fibrosis or other lung pathology (not obstructive pathology). Thus, the FEV1/FVC ratio should be approximately normal, or even increased due to a decrease in magnitude of FVC as compared to FEV1 (because of the decreased compliance associated with the presence of fibrosis in some pathological conditions)

Obstructive vs restrictive disease on graph
Note here the x-axis represent not the volume in the lung but the volume that is expired. These axis are labelled either way in different text books so please make sure to pay attention to this.



