L18 One Carbon Metabolism Flashcards
Overview:
- What is one carbon metabolism?
- What amino acids are the carbon donors?
- What is the carrier molecule?
- What are th two types of reactions modified folate participates in?
- One carbon metabolism is a network of integrated metabolic pathways act together to continually
supply single-carbon units needed for various biochemical reactions. - The amino acids serine, glycine, and histidine can serve as initial donors of one-carbon units. Serine is the major donor of one-carbon units in the
body. - The donated carbon unit is first bound to a carrier molecule (a modified form of folate)
- a. Folate can directly donate its one-carbon unit to nucleic acid intermediates in the synthesis of nucleotides (purines or thymidine).
- b. Folate can also donate its one-carbon unit to a second type of onecarbon carrier molecule, homocysteine. This carrier is further modified and eventually donates its one-carbon methyl group for methylation reactions.
Reaction Pathway Overview:
Folate undergoes a series of reductions and accepts C, some of the intermediates are needed for purine and dTMP synthesis:
folate→dihydrofolate(DHF)→terahydrofolate(THF)
THF→N10formyl-THF (used in purine synth) →
→ serine donates C→
→ N5-N10-methyleneTHF (used in dTMP synth) →N5-methylTHF
Then Vitamin B12 dependent Homocysteine Methyltransferase:
Transfers C from N5-methylTHF to methionine, recycling THF and creatin SAMe:
Methionine→SAdenosylmethionine(SAMe) donates CH3 to the target accepter
SAMe goes through a series of reactions to produce Homocysteine which can be recycled to methione or THF via Homocystein Methyltransferase
SAMe→S Adenosylhomocysteine(SAH) →
→ Homocysteine → methionine or THF

- What is folate?
- What are it’s components?
- What is the source of folate?
- What is the most common cause of folate deficiency?
- How does heat affect folate?
- The group of compounds consisting of folic acid and its derivatives are collectively referred to as folates.
- Folic acid, also called pteroyl glutamate or folyl monoglutamate, is composed of three parts: a pteridine ring, p-aminobenzoic acid (PABA), and one glutamic acid residue.
-
Fruits and vegetables are the main source of
folate in the human diet. - Insufficient dietary intake is the most common cause
of folate deficiency. - Folate is heat-labile and easily destroyed by
cooking (50-90% of the vitamin depleted).
- What form is dietary folate usually in?
- How is it named?
- How is folate inititally modified?
- Dietary folate (mainly from fruits and vegetables) is present largely as conjugates, in which folate is bound to multiple glutamic acids residues.
- Conjugates are named according to the length of the glutamate chain (pteroyl + (mono-, di-, tri-, etc) glutamate).
- In humans, glutamic acids can be removed from folate by the action of deconjugating enzymes (conjugases), neccessary for absorption.
- Where is folate deconjugated?
- How is it absorbed?
- What is the primary form of folate in circulation?
- A brush-border conjugase on the enterocyte surface systematically removes single glutamate residues from the end of the glutamate chain, ultimately yielding folyl monoglutamate (aka folic acid or pteroyl glutamate).
-
Folyl monoglutamate is actively transported into the enterocyte via a reduced folate carrier protein. Within the enterocyte, folic acid is metabolized to N5-methyl
tetrahydrofolate (N5-methyl-THF) and then released into the circulation. - N5-methyl-THF (a modified form of folate with one glutamic acid residue) is the primary form of folate in the circulation.
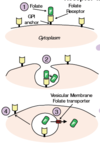
How is folated taken up into tissue from circulation?
4 steps:
Receptor-mediated Endocytosis of Folate:
- GPI-anchored folate receptors (on
the cell plasma membrane) bind to N5-
methyl-THF in the circulation with high
affinity. - Folate undergoes receptormediated
endocytosis and is subsequently
released into the lumen of the acidified
vesicle within the cytoplasm. - Folate is then moved from the
lumen of the vesicle into the cytoplasm of
the cell via a vesicular membrane folate
transporter. - The vesicles containing folate
receptors are recycled to the cell surface,
where the receptors can once again take up
folate.
Metabolic Pathway of Folate
Folic acid first undergoes two
NADPH-dependent reductions
(via the enzyme dihydrofolate
reductase) to form the active
form of folate, tetrahydrofolate
(THF).
Binding to THF allows onecarbon
compounds to be
recognized and manipulated by
biosynthetic enzymes.
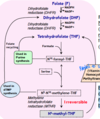
How does folate contribute to nucleotide synthesis?
N10-formyl-THF and N5N10-methylene-THF are important carriers of one carbon units for the synthesis of purines (adenine and guanine) and dTMP (thymidine).
After the removal of its one-carbon unit, folate itself can be metabolized back to either DHF or THF. Conversion of DHF to THF is dependent on the activity of the enzyme dihydrofolate reductase.
- *The N5N10-methylene** form of THF can also be metabolized by the enzyme methylene tetrahydrofolate reductase (MTHR) to form N5-methyl-THF. (this form is the carbon donor)
- *The reaction catalyzed by MTHR is irreversible.**
The drug methotrexate is a competitive reversible inhibitor of dihydrofolate reductase, and is used in chemotherapy regimens for a variety of cancers.

What is methotrexate?
Methotrexate, a structural analog of folic acid, inhibits the enzyme dihydrofolate reductase, reducing the ability of the cell to recycle folate. The inhibition of folate recycling causes an associated decline in the synthesis of dTMP and purines, which are most needed by rapidly-dividing cells synthesizing new DNA (erythroblasts/cancer).

How can decreased folate metabolism contribute to Megablastic Anemia?
Asynchrony of cell growth as well as the decreased proliferation and death of erythropoietic cells can result in a condition called megaloblastic anemia. (cells get too big from continued protein synthesis w/out cell division)
Rapidly-dividing cells are very sensitive to folate deficiency related declines in nucleotide synthesis. The decreased availability of nucleotides slows DNA synthesis, leading to an asynchrony between DNA synthesis and protein production, and resulting in large cells.
Erythroid cells rapidly divide in the course of their development, and are the first to exhibit pathology when folate levels are abnormally low or folate
metabolism is compromised.
In addition, when folate levels are low, dTTP is scarce but dUTP is plentiful and can become inappropriately incorporated into DNA. This results in an over-activation of dUTP-directed DNA repair mechanisms which introduce
DNA strand breaks and can results in higher levels of cell death.
What are the characteristics of Megaloblastic Anemia in a peripheral blood smear?
Oval macrocytes: These are large, oval, fully
hemoglobinized erythrocytes.
Marked anisocytosis (unequal size) and
poikilocytosis (varied shape); high RDW
Low or normal reticulocyte count (CR) due to
decreased erythropoiesis. (note: RC count is elevated w/ anemia but not w/ megaloblastic anemia = way to distuingish)
Large hypersegmented neutrophils: Greater
than 5 lobes in the nuclei is abnormal, and is
likely due to cell division/DNA synthesis defects.
Thrombocytopenia (decreased platelet number)
and leukopenia (decreased white cell number)
What are the characteristics of a bone marrow smear with Megaloblastic Anemia?
Can peripheral blood smears or bone marrow smears distinguish between a Vit B12 deficiency and folate deficiency?
Megaloblastic anemia displays
megaloblastic changes in all stages of red cell development. Decreased DNA synthesis leads to hypoproliferation and the premature destruction of
megaloblasts within the bone marrow(ineffective erythropoiesis).This is the cause of the observed anemia.
Development of cells from other lineages, including white blood cells, can also be affected. Increased destruction of platelet precursors (including megakaryocytes) leads to thrombocytopenia (too few platelets).
Increased destruction of
granulocytes (white blood cell
precursors) leads to leukopenia
(decreased white cell count).
_Note: These morphology changes
cannot be used to distinguish a
folate deficiency from a vitamin B12
deficiency as the cause. This will be
addressed in the workshop._
What is the difference in morphology between cells with microcytic and megaloblastic (macrocytic) anemia?
Macrocytic has large cells from continued protein production w/out cellular division, caused by folate or vitamin B12 deficiency.
Microcytic anema has smaller cells from a lack of protein (hemoglobin) production, in example, iron deficiency.
What is the role of folate in methylation?
N5-methyl-THF can donate its methyl group to a second type of onecarbon carrier, homocysteine.
Vitamin B12 (also known as cobalamin) functions as a cofactor for the enzyme homocysteine methyltransferase.
Homocysteine methyltransferase takes the donated methyl group from N5-methyl-THF and binds it to vitamin B12, which is a cofactor bound to the enzyme. This forms methylcobalamin. Then the enzyme transfers the methyl group off of vitamin B12 and onto homocysteine, forming methionine.
The products of this reaction are methionine (used to ultimately provide one carbon units for methylation reactions) and THF, which can be recycled for use in folate-dependent reactions.

What are the two reasons Homocysteine methyltransferase is vitally important in the cell?
- Metabolizes N5-methyl-THF into THF, a form of folate that can again be used as a one-carbon carrier.
Note: N5-methyl-THF cannot be used to supply one-carbon units for nucleotide synthesis and MUST be metabolized back to THF to restore its bioavailability.
-
Facilitates the transfer of the one-carbon (CH3) group from N5-methyl-THF to homocysteine, forming methionine. This step is essential for the synthesis of the terminal methyl donor, S-adenosyl
methionine (SAMe).




