Innate Immunity Flashcards
Innate Immune System
Definition
Set of host defense mechanisms that are always in place to provide early protection against microbial infections.
Innate Immunity
Functions
- Controlling infection and in some cases eliminating microbial pathogens prior to any symptom onset
- Facilitating the initiation and development of pathogen-specific adaptive immune responses
- Cooperating with adaptive immune defenses to effectively eliminate microbial pathogens
- Removes damaged tissues and promote repair
External Defenses
Physical and Mechanical Barriers:
- Stratified squamous epithelium
- Mucus layer
- Mucocilliary escalator
- Peristalsis
- Normal Flora
Chemical and Biochemical Barriers:
- Sweat and sebaceous secretions
- Lactic acid
- Fatty acids
- Waxes
- Alcohols
- Gastric acidity
- Digestive enzymes
- Bile salts
- Lysozyme
- Lactoferrin
- Transferrin
Internal Defenses
Cellular Components:
- Neutrophils
- Monocytes/macrophages
- Natural killer cells
Soluble Components:
- Complement system
- Cytokines
- Chemokines
- Acute phase proteins
Innate Immunity
Recognition
- Recognize repeating patterns of molecular structure that are common to certain classes of pathogens
- Structures are not expressed by host cells and signal the presence of non-self or foreign antigens
- Shows coarse specificity
Pathogen Associated Molecular Patterns
(PAMPs)
- Conserved molecular structures common to classes of pathogens
- Often part of essential structures with limited variability
- Recognized by cells of the innate immune system

Damage Associated Molecular Pattern Molecules
(DAMPs)
- Molecules generated or released following tissue damage
- Induced by microbial infection
- Induced during a non-infectious inflammatory response where cells are damaged or stressed
- Trauma
- Burns
- Chemical toxic exposure
- Ischemia/reperfusion injury
- Released during necrotic death but not apoptotic death
- Recognized by the innate immune system

Pattern Recognition Receptors
(PRR)
- Innate immune system receptors for PAMPs and DAMPs
- Germ-line encoded (no somatic recombination)
- Limited repertoire compared to T/B cell receptors
- Are not clonally distributed
- Present on all cells of the same lineage
- I.E. all macrophages have a certain type regardless of location
- Families of PRR’s exist to respond to specific treats i.e. extracellular, cytosolic, and endosomal classes
-
Cell-associated PRR include:
- Toll-like receptors
- Scavenger receptors
-
Soluble recognition molecules include:
- Collectins
- Collagen-containing carbohydrate binding proteins
- Complement
- Collectins
-
Cell-associated PRR include:

Toll-Like Receptors
- Prototypical type of PRR
- Set of receptors on cytoplasmic and endosomal membranes
- After binding ligand will dimerize to transduce a signal
- Have relatively conserved cytoplasmic tails which activate common adaptor proteins

Toll-like Receptor
Activation
Functions through two common adaptor proteins:
-
MyD88
- Activates NF-𝛋B transcription factors
- Turns on genes associated with:
- proinflammatory response
- cytokine response
-
TRIF
- Activates IRF transcription factors
- Turns on Type 1 interferon genes
- Important in viral infections

Activated PRR
Functions
- phagocytosis and killing of the organism
- recruitement of immune cells to the site of infection
- production of effector molecules that:
- limit pathogen growth
- recruit and activate additional immune cells to the site of infection (T/B cells)
- influence the development of the adaptive immune response
- tissue repair and remodeling
Innate Immune
Defense Against Bacteria
(Extracellular Pathogens)
- Phagocytes
- IFN-γ
- Complement
- Inflammation
Neutrophils
(PMNs)
- Short-lived cell
- Predominant WBC of peripheral blood
- Contains numerous granules which fall into two categories:
- Primary (Azurophilic) granules: contain myeloperoxidase and cationic proteins
- Secondary (specific) granules: contain lysozyme and lactoferrin
- First cells to arrive at an inflammatory focus (~6-12 hrs)
- Major defense against pyogenic bacteria:
- Staphylococcus
- Streptococcus
- Neisseria
Mononuclear Phagocytes
Monocytes/Macrophages
- Larged long-lived cells
- Monocytes in blood ⇒ macrophages in tissue
- Assume different names depending on which tissue they reside in
- Resident macrophages provide sites of filtration where microorganisms can be removed.
- Circulating monocytes move from blood into tissue in response to infection and inflammation.
- Arrives after neutrophils (~12-24 h)
- Due to ability to become “activated” they are more potent effector cells
Dendritic Cells
- Located in peripheral tissues
- Phagocytic capabilities
- Antigen presenting cells
- Helps to activate T-cells and initiate the adaptive immune response
Phagocytosis
- Dependent on cell-surface receptors which mediate attachment of the organism to phagocytes
- Examples:
- Macrophage mannose receptors
- Scavenger receptors
- Receptors for antibodies
- Receptors for complement components
-
C3b: can be deposited on bacterial cell surfaces following activation of complement by the alternative pathway in the early innate immune response to bacterial infection
- Binds to CR-1 on phagocytes
- Mac1 receptors can bind iC3b or C4b
-
C3b: can be deposited on bacterial cell surfaces following activation of complement by the alternative pathway in the early innate immune response to bacterial infection
- Examples:
- Phagocytes engulf the organism and enclose it in a phagosome
- Becomes increasingly acidic
- Fuses with cytoplasmic granules to form phagolysosome
- Granule contents discharged around ingested microbe
- Subsequent intracellular response includes:
- Oxygen independent
- Oxygen-dependent pathways
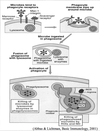
Oxygen-Independent
Antimicrobial Mechanisms
- Defensins
- Cationic proteins
- Cathepsin G
- Lysozyme
- Bactericidal permeability increasing (BPI) proteins
- Proteolytic enzymes
- Lactoferrins
- DNAses
- Acid hydrolases
Oxygen-Dependent
Antimicrobial Mechanisms
Occurs in macrophages and neutrophils
- Respiratory Burst is a vigorous burst of oxygen consumption following activation
-
NADPH-dependent oxidases generate reactive oxygen intermediates (ROI)
- Superoxide anion (O2-)
- Hydrogen peroxide (H2O2)
- Singlet oxygen (O2)
- Hydroxyl radicals (OH-)
- ROI are potent anti-microbials
- Can also cause local damage to host
- So system is down-regulated quickly
Neutrophils only
- Neutrophils also have myeloperoxidase
- Use hydrogen peroxide and Cl- to produce a halogenating system (OCl- or hypochlorite anion) = bleach

Reactive Nitrogen Intermediates
(RNI)
- Occurs as part of the respiratory burst
- Nitric oxide (NO) produced from arginine and oxygen by nitric oxide synthase
- NO reacts with oxygen radical to produce peroxynitrite and reactive nitrogen intermediates (RNI)
- Peroxynitrite causes damage to cell walls and viral capsules
Macrophage
Gram-negative Response
- Lipopolysaccharide (LPS) of gram-negative bacteria binds to serum LPS-binding protein to form LPS+LPS-BP complex.
- LPS+LPS-BP complex binds the CD14 receptors on surface of monocytes/macrophages.
- LPS-CD14 receptor complex interacts with cell-surface Toll-like receptor-4 (TLR-4) leading to cell activation.
- Stimulates cytokine synthesis and release:
- TNF-α
- IL-1
- IL-6
- IL-8
- IL-12
- Cytokines important for:
- Recruitment of effector cells
- Lymphocyte activation & differentiation

Interferon-γ
Type II Interferon
- Secreted by NK cells early as part of the innate immune response
- Secreted by T cells late as part of the adaptive immune response
- Extremely important in the recruitment and activation of macrophages
- To phagocytize and kill microbial pathogens
- To secrete additional cytokines, chemokines, and antimicrobial products
- Important in the stimulation of adaptive immune responses and influence the nature of that response
- Example:
- Tuberulosis lives within modified vacuoles of macrophages
- Macrophages can be induced by IFN-γ to kill intracellular pathogens
Defense Against Viruses
- Initial production of cytokines including
- IFN-α and IFN-β by viral infected cells
- TNF-α and IL-12 by macrophages
- Subsequent NK cell activation and killing of virally infected cells
- Development of the adaptive immune response with virus specific:
- T-cells that kill virally infected cells
- B-cells that secrete antibodies that can block viral entry into host cells
Type I Interferons
Basics
- Large family of structurally related cytokines
- Mediate the early innate immune response to viral infections
- Able to interfere with viral replication
- Most significant are IFN-α and IFN-β
Type I IFN
Induction
- Viral nucleic aids bind to intracellular receptors linked to the production of transcription factors
- TLR-3
- RIG-1
- Toll-like receptors can be found on endosomal membranes that recognize dsRNA and ssRNA
- Cytoplasmic sensors recognize viral RNA
- Stimulation of either causes activation of interferon regulatory transcription factors
- Within several hours of a viral infection, host cells begin to produce and secrete IFN-α and/or IFN-β
Type I IFN
Effector Functions
-
Transcription Regulation
- All type I IFN’s bind to the same cell surface receptors
- Signals host cell to activate or increase synthesis of a large set of proteins
- Net result is an increased resistance to viral replication in all cells
- Stimulation of effector mechanisms to kill virally infected cells
-
Anti-viral Functions
- IFN α/β induced proteins can directly inhibit one or more steps in the viral life cycle (entry, transcription, translation, assembly, release)
-
Promotes viral genome degradation inside host cell
- Activates oligoadenylate synthetase
- Polymerizes ATP into 2’,5’ linked oligomers
- Activates endoribonuclease (RNase) to degrade viral RNA
- Activates oligoadenylate synthetase
-
Inhibits viral protein synthesis
- Activates P1 kinase (ser/thr kinase)
- Phosphorylates eIF-2
- Leads to inhibition of all protein synthesis
- Activates P1 kinase (ser/thr kinase)
-
Immuno-regulatory functions
- Increases MHC expression, viral antigen processing, and presentation to virus-specific T cells
- Helps to initiate the adaptive immune response
- Activates NK cells to kill virus infected host cells
NK cells
Basics
- Large granular lymphocytes
- Distinct from T cells and B cells
- Found in blood and spleen
- Migrates into infected tissues in response to inflammatory cytokines
- After recognition, able to kill various targets without need for additional activation
- Function can be enhanced by:
- IFN α/β initially produced in response to a viral infection
- Cytokines produced by macrophages early in the course of many infections:
- Interleukin-12 (IL-12)
- Tumor necrosis factor-α (TNF-α)
NK Cells
Recognition Methods
NK cells do not express an antigen specific receptor.
- Express a set of activating and inhibitory receptors.
- Cooperate to allow the recognition of many virally infected host cells and tumor cells
- Example:
- Viruses down-regulate MHC class I receptors to evade T-cells
- Lack of MHC class I causes activation of NK cells
- Express an IgG binding FC receptor (CD16) on surface
- Facilitate Antibody Dependent Cellular Cytotoxicity (ADCC)
- IgG binds to surface of virally infected cells
- Facilitates NK cell recognition

NK Cell
Cytotoxicity
Important in the early control of viral infections and mediate the killing of virus infected cells.
- After activation, NK cell cytoplasmic granules are discharged onto the surface of virally infected cells.
- Granule proteins (Perforin) create a pore in the plasma membrane of infected cells.
- Allows entry of granzymes which activates an apoptotic cascade.
- Leads to host cell death.
Also able to kill through the Fas:FasL pathway.
(Discussed later)
NK Cell
Cytokine Production
Activated NK cells secrete cytokines including:
- Interferon-γ
- Important in the recruitment and activation of macrophages
- Helps shape the cytokine profile secreted by TH cells
Innate & Adaptive Immune System
Interactions
Cooperation between adaptive and innate immune systems can enhance the effectiveness of the overall response.
- Antibody-dependent cellular cytotoxicity (ADCC)
- NK cells bind Fc portion of IgG
- Facilitates NK cell identification and killing of targets prior to the release of cytotoxic mediators
- Opsonization
- IgG or complement components (C3b) coat surface of pathogens
- Phagocytes express Fc receptors of IgG and C3b receptors
- Opsonized antigen phagocytized more readily
- Classical pathway of complement activation


