Immunology Flashcards
Define innate immunity and recall the host-microbe interactions
Innate immunity:
- First line (and second line) of defence against invading pathogens
- First line: skin, mucous membranes, secretions of skin and mucous membranes
- Second line: phagocytic leukocytes, antimicrobial proteins, inflammatory response
- Can activate acquired immune system
- Ability to differentiate between host and pathogen (e.g. sentinel cells in stratum spinosum)
Host-microbe interactions – (bacterial) infection pyramid:

List the main functions of the innate immune system (5 points)
Functions of innate immune system:
- Anatomic and physiologic barriers
- Phagocytosis
- Activation of acquired immune system
- Immune cell recruitment
- Completement activation
List the anatomic and physiologic barriers of the innate immune system
Anatomic and physiologic barriers of innate immune system:
“_S_avage _M_ermaids _S_mash _T_urtles”
- Skin – physical barrier; tight junctions in epithelium; desquamation to remove adhered pathogens
- Mucous membranes – resp. and GI tracts; slimy surface traps pathogens, ciliary clearance; defensins
- Secretions – stomach acid, digestive enzymes, tears (lysozyme), mucous, sweat
- Temperature – Normal body temperature and/or fever inhibits growth of some pathogens.
Describe the function of defensins in the innate immune system
Defensins:
- Contained in mucous membranes of all animal and plant cells
- Short, +ve charge (cationic) proteins, with hydrophobic and amphiphilic domains
- Abundant in neutrophils (to kill phagocytosed pathogens)
- Broad spectrum of antimicrobial activity (Gram +ve/-ve bacteria, fungi, parasites, enveloped viruses).
- Difficult for microbes to acquire resistance to defensins.

What is the overall host response to injury or infection?
Host response to injury or infection – the inflammatory response.
Inflammatory response has two main immunological components:
- Innate, non-adaptive response (non-specific)
- Acquired, adaptive response (specific response)
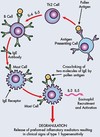
Outline the stages of the innate immune response against injury or infection
Stages of the innate immune response:
- Occurs immediately on injury or infection
- Comprises vascular and cellular changes
- PRRs on sentinel cells detect PAMPs on pathogens – triggers cytokine release (IL-1, TNF-α):
- vasodilatation
- expression of adhesion molecules on cell surfaces
- Leukocytes roll on, adhere to and migrate through activated vascular endothelium towards pathogen
- Phagocyte (leukocyte) and NK cell (lymphocyte) activity
- Complement system activation (end result is cell lysis via MAC complex)
- Activation of acquired immune response
Describe and explain the role of pathogen associated molecular patterns (PAMPs) and pattern recognition receptors (PRRs)
Recognizing a pathogen – PAMPs and PRRs
Pathogen associated molecular patterns (PAMPs):
- Microorganism surface components
- Peptidoglycan cell wall
- Flagella of bacteria
- Lipopolysaccharide (LPS) on Gram -ve bacteria
- Teichoic acids on Gram +ve bacteria
- Molecules in the cell walls of fungi (zymosan, glucan, chitin)
- Short bacterial DNA sequences (CpG motif – twenty times less common in vertebrate DNA than in bacterial DNA)
PAMPs are specific to pathogens (pathogen-associated) and enables the immune system to distinguish self from non-self via host pattern recognition receptors.
Patten recognition receptors (PRRs)
- Soluble receptors in cytoplasm:
- NOD-like receptors (NLRs)
- RIG-I-like receptors (RLRs)
- Membrane-bound receptors:
- Toll like receptors (TLRs)
- C-type lectin receptors (CLRs)

Recall the 6 main outcomes TLR stimulation

Which TLRs recognize bacterial lipids?
11 families in mammals (TLR 1–11):
TLR 1, 2, 4, and 6 recognize bacterial lipids
Which TLRs reconize viral RNA?
TLR 3, 7, and 8 recognize viral RNA
Which TLRs recognize bacterial DNA?
11 families in mammals (TLR 1–11):
TLR 9 recognizes bacterial DNA
Which TLRs recognize bacterial and parasite proteins?
11 families in mammals (TLR 1–11):
TLR 5 and 10 recognize bacterial and parasite proteins
Which TLRs are present on the cell surface?
Which TLRs are intracellular?
Cell surface: TLR 1, 2, 4, 5, 6, and 10
Intracellular: TLR 3, 7, 8, 9, and 11
Describe the stages involved in phagocytosis (7 points)
Stages of phagocytosis:
- Binding of opsonins (complement or antibody) to pathogen (or antigen presenting cell)
- Phagocyte recognizes opsonin +/- PAMPs of pathogen
- Cell membrane of phagocyte extends and envelops opsonized target
- Pinches off in cell cytoplasm to form a phagosome
- Phagosome fuses with lysosome to form phagolysosome
- Lysosomal enzymes digests contents
- Release of digestion products from cell
TLR-mediated signalling pathways lead to the translocation of transcription factors that activate the elements of immune response

List 3 types of phagocyte
Phagocyte:
- Monocytes
- Dendritic cell
- Macrophage (Mφ)
- Neutrophil
- Mast cell
List the 3 distinctive pathways of the complement system
Distinct pathways of the complement system (3 points):
- Classical pathway (Ag-Ab)
- Lectin pathway (microbial carbohydrates: MBL, MASP-1, -2)
- Alternative pathway (spontaneous, activating surfaces)
All meet at level of C3 convertase which reacts with C5 to produce membrane attack complex (MAC)
Describe the action of natural killer cell (NK cell)
NK cells cause lysis of targets cells via induction of apoptotic or necrotic pathways
Action of NK cell depends on balance of activating and inhibitory signals via receptors on NK cell:
- Activating receptors recognize molecules that are expressed on the surface on cancer cells and infected cells, and ‘switch on’ the NK cell
- Inhibitory receptors on the surface of NK cell recognize cognate MHC I (found on normal healthy cells), and this ‘switches off’ the NK cells, prevent it from killing.
Recall the mechanism of action of NK cell (4 points)
Cancer cells and infected cells often lose their cell surface MHC I, leaving them vulnerable to NK cell killing (since MHC I normally inhibits NK cells).
Mechanism of action of NK cell (4 points):
- NK cell releases cytotoxic granules containing perforin and granzymes
- Perforin creates pore in cell membrane of target cell (e.g. pathogen, tumor)
- Granzymes enter cell cytoplasm
- Granzymes induce apoptosis

Define acquired immunity
Acquired, adaptive immune response (specific response):
- Immunity that an organism develops during lifetime – after exposure to antigens
- Involves activity of lymphocytes
- Includes 3rd line of defence (1st and 2nd line involved in the innate, non-specific immune response)
Recall the different types of passive vs active acquired immunity
Acquired immunity may be acquired either actively or passively:
List the 4 key attributes of acquired immune response
Acquired immune response exhibits 4 immunologic attributes:
- Specificity
- Diversity
- Memory
- Self/non-self recognition
Explain why acquired immunity elicits a specific, later response than innate immunity
The acquired immune response is slower-acting, longer-lasting, and more specific than the innate immune response:
- The acquired immune response occurs in two phases: the induction phase and effector phase
- The acquired immune response first requires signalling from the innate immune response to function
- Antigen presenting cells (APCs) of the innate immune system, e.g. macrophages and dendritic cells, display antigens via MHC molecules to complementary naïve T lymphocytes e.g. CD4+ (MHC II) or CD8+ (MHC I), of the acquired immune system.
- In response, the T lymphocytes need time to differentiate and proliferate into TH cells or Tc cells (i.e. the induction phase).
Therefore, the process of responding to the APCs of the innate immune system takes time because it involves protein synthesis and cell division, but lasts much longer and has greater specificity.
Which lymphocytes are involved in cell-mediated immunity?
Which lymphocytes are involved in antibody-mediated immunity?
T lymphocytes – involved in cell-mediated immunity (i.e. cellular immunity)
B lymphocytes – involved in antibody-mediated immunity (i.e. humoral immunity)
Describe the key features and functions T lymphocytes:
- Expression of cell surface receptors and secretion of proteins
- Structure of cell surface receptors and secretion of proteins
- Diversity of cell surface receptors and secretion of proteins
T lymphocytes (matured in the thymus):
- T cell receptor (TCR) – protein complex which detects antigen bound to MHC I or MHC II:
- Class I MHC proteins – found on virtually all body cells (including cancer cells)
- Class II MHC proteins – found on certain cells in the immune response (APCs)
- T lymphocyte types (diversity):
- Helper T cell (TH) – secrete cytokines to help B cells and Tc cells to divide
- Cytotoxic T cells (Tc , or killer T cells) – kill infected body cells
- Memory T cells (MT) – remain in the body in case of subsequent exposure to antigen.
Described the key features and functions B lymphocytes:
- Expression of cell surface receptors and secretion of proteins
- Structure of cell surface receptors and secretion of proteins
- Diversity of cell surface receptors and secretion of proteins
B lymphocytes (matured in the bone marrow):
- B cell receptor (BCR) – antibodies (i.e. immunoglobulins, Ig) can detect extracellular antigens
- IgD is the antibody specific to B lymphocytes (B cell activation, can’t cross placenta)
- Behave directly as APCs, and differentiate into plasma cells (release Ab) and memory B cells (MB).
- B lymphocyte types (diversity):
- B cell which differentiates into plasma cells (release antibodies)
- B cell which differentiates into memory B cells (MB cells) which remain in the body in case of subsequent antigen exposure.
Explain the role of B lymphocytes in humoral immunity
Antibody-mediated (humoral) immunity:
- Target extracellular microorganisms (bacteria and viruses circulating in blood)
- TH cells stimulate B cells that have engulfed and presented pathogen-derived antigens.
- B cells differentiate into plasma cells that secrete antibodies.
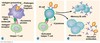
Identify the immunoglobulin classes (IgG, IgA, IgM, IgD, IgE) and their functions

Explain the nature of immunoglobulin-antigen binding
Immunoglobulin (antibody) nature:
- 2 variable ‘arms’ regions specifically for antigen binding (antigen-binding fragment, Fab)
- 1 constant ‘tail’ region specifically for cells of immune system (constant fragment, Fc).
‘Y-shape’ Ig

Differentiate between the primary and secondary phases of the humoral immune response (8 step explanation)
- First ever exposure to antigen (e.g. pathogen): innate immune system recognizes antigen as foreign (sentinel cell phagocytoses pathogen, presents antigen protein fragments – now considered APC).
- APC interacts with CD4+ and CD8+ T lymphocytes via the APC’s MHC I and MHC II surface proteins, respectively. Induction phase begins:
- CD4+ and MHC II interaction initiates TH cell proliferation and B cell proliferation (humoral response)
- CD8+ and MHC I interaction initiates proliferation of Tc cell proliferation (cellular response)
- Predominant antibody type in primary response to first ever antigen exposure: IgM (pentameric)
- Both interactions also produce memory T cells and memory B cells ready for subsequent exposure
- Subsequent exposure to the same antigen initiates a more rapid secondary response (since there is no need for innate immune response) through activation of memory B cells (act directly as APC) and T cells into effector cells.
- Predominant antibody type in secondary response: IgG (monomeric)

Explain how immunoglobulins (antibodies) protect against infectious agents, including:
- Neutralisation
- Agglutination
- Opsonisation
- Activation of complement
- Antibody-dependent cell-mediated cytotoxicity
Neutralization – antibodies block the activity of the pathogen by saturating pathogen’s cell surface antigens.
Agglutination – multiple pathogens are aggregated by antibody molecules (macrophages then phagocytose).
Opsonization – pathogens bound by antibodies are more efficiently engulfed by phagocytes (free Fc region).
Activation of complement – antibodies bound to pathogens activate the complement cascade (free C1 protein binds to Fc region of antibody)
Antibody-dependent cell-mediated cytotoxicity – abnormal body cells that are bound by antibodies are recognized by NK cells and are subsequently lysed (free Fc region).
Describe the three complement pathways (classical, alternative and lectin)
Classical pathway:
- Ag-Ab complex on pathogen surface (C1q,r,s)
MB-Lectin pathway:
- Mannose-binding lectin binds mannose (sugar monomer) on pathogen surface
Alternative pathway:
- Spontaneous hydrolysis of C3-H2O

Describe the extracellular (exogenous) antigen processing pathway by APCs (4 points)
Extracellular (exogenous) antigen processing by APC:
- Exogenous antigen is ingested by phagocytosis (trapped inside intracellular phagosome)
- Phagosome combines with lysosome to form phagolysosome (contains lysozymes)
- Lysozymes and acidic environment of phagolysosome degrade antigen into small peptides
- Small peptides are then presented with class II MHC molecules on cell membrane of APC.

Describe the intracellular (endogenous) antigen processing pathway by APCs (4 points)
Intracellular (endogenous) antigen processing by APC (4 points):
- Endogenous antigen is produced inside the cell itself (e.g., in a virus-infected cell)
- Antigen is degraded within the cell cytoplasm into small peptides
- Small peptides move into endoplasmic reticulum and bind to class I MHC molecules
- Peptide-class MHC I complexes then move through Golgi apparatus to cell membrane of APC.

Define major hisocompatibility complex (MHC)
Major histocompatibility complex (MHC) molecules:
- Major (i.e. essential)
- Histo- (relating to tissues)
- -Compatibility (genetic variation so different shape protein; leads to non-self rejection of tissues).
Two main classes of MHC:
- Class I MHC
- Class II MHC
Explain the role of MHC molecules in adaptive immunity
Class I MHC presents antigen and interacts with complementary CD8+ T lymphocytes
- MHC I-TCR complex is stabilized by CD8+ peptide (co-receptor) to allow for IL-1, -2 signalling
- Initiates cell-mediated immune response (proliferation of Tc cells)
Class II MHC presents antigen and interacts with complementary CD4+ T lymphocytes
- MHC II-TCR complex is stabilized by CD4+ peptide (co-receptor) to allow for IL-1, -2 signalling
- Initiates cell-mediated (proliferation of TH cells, Treg cells) and antibody-mediated immune response (proliferation of B cells then differentiation into plasma cells)
Explain how helper, cytotoxic and regulatory T cells contribute to cell-mediated immunity
Cell-mediated immunity (immune response towards infected cells, cancer cells and transplant cells):
- Helper T cells – secrete cytokines and help B cells and Tc cells to divide
- Cytotoxic T cells – kill infected body cells by perforin and granzyme action
- Regulatory T cells – restrain and inhibit the cell-mediated response to prevent excessive reaction and autoimmunity
Explain how helper T cells regulate the immune system
Helper T cells (TH):
- Bind to other white blood cells that have previously encountered an antigen and release cytokines:
- Stimulate proliferation of other T cells
- Stimulate B cells that have already become bound to antigen
- Without TH cells there is no immune response
Explain the function of cytotoxic T cells
Cytotoxic T cell (Tc):
- Destroys infected body cells
- binds to target cell
- secretes perforin protein (punctures cell membrane) and granzymes (induces apoptosis).
Describe the role of regulatory T cells
Two main types of regulatory T cells:
- Inducible regulatory T cells (iTreg) are derived from T helper cells precursor (TH0)
- Naturally occurring regulatory T cells (nTreg) are matured in the thymus
Regulatory T cells restrain and inhibit the development of the immune response, preventing autoimmunity and excessive immune activation.
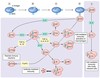
Recall the overall picture of the innate and adpative immune responses
List the 3 basic layers of the mucosa
Mucosa made up of 3 basic layers:
- Epithelial lining (innermost, luminal aspect)
- Lamina propria (cellular connective tissue deep to epithelial layer, e.g. lymphocytes)
- Muscularis mucosae (deepest aspect of mucosa; enables peristalsis of mucosa)
Summarize the lymphatic system
The lyphatic system:
- The immune system is organised into several specialized tissues which are collectively termed as lymphoid or immune tissues
- Tissues that have evolved to a high degree of specificity of function are termed as lymphoid organs
- Organs of the immune system have been divided into:
- Primary lymphoid tissue
- Secondary lymphoid tissue

List the 2 primary (central) lymphoid tissues
Primary (central) lymphoid tissues:
- Bone marrow
- Thymus
List the 3 secondary (peripheral) lymphoid tissues
Secondary (peripheral) lymphoid tissues:
- Lymph nodes
- Spleen
- MALT
Describe the structure of lymph nodes (5 points)
Structure of lymph node (5 points):
Surrounded by a fibrous capsule from which trabeculae penetrates the nodes
- Outer cortex – accumulation of lymphocytes (primary lymphoid follicles) within which germinal centres (secondary follicles) develop during antigenic stimulation. Follicle also contain dendritic macrophages.
- Inner medulla – lymphocytes, plasma cells and macrophages are arranged as elongated branching bands (medullary cords).
- Bursa dependent areas – the cortical follicles and medullary cords that contain B-lymphocytes.
- Thymus dependent area – between the cortical follicles and medullary cords there is an ill-defined intermediate zone (paracortical area) which contains T lymphocytes.

List the key functions of lymph nodes (4 points)
Functions of lymph node:
- Filter for lymph, each group draining specific part of the body.
- Phagocytose foreign materials including microorganisms.
- Help in proliferation and circulation of T cells and B cells.
- They enlarge during local antigenic stimulation.
Describe the structure of the spleen
Structure of the spleen
Largest lymphoid organ which has a capsule from which trabeculae descend, dividing the organ into several interconnected compartments. The spleen contains two main tissue types: white pulp and red pulp.
- White pulp – lymphatic tissue (material which is part of the immune system) mainly made up of white blood cells, constitute 75% of the organ.
- Red pulp – made up of blood-filled cavities (venous sinuses) and splenic cords. Splenic cords are special tissues which contain different types of red and white blood cells.