Immunology Flashcards
What are the constitutive barriers to infection?
- Skin
- Mucosal surfaces
- Commensal bacteria
How does the skin act as a constitutive barriers to infection?
- Consists of tightly packed keratinised cells - physically limits colonisation by microorganisms
- Physiological factors - low pH and low oxygen tension
- Sebaceous glands - produce hydrophobic oils, lysozymes and ammonia
How does the mucosal surfaces act as a constitutive barriers to infection?
- Mucus = physical/trapping barrier - contains secretory IgA, lysozymes, antimicrobial peptides and lactoferrin
- Cilia directly trap pathogens - assisted by physical manoeuvres (sneezing/coughing)
How does the commensal bacteria act as a constitutive barriers to infection?
- 100 trillion bacteria normally reside at the surfaces
- Compete with pathogenic bacteria for scarce resources
- Produce fatty acids and bactericidins - inhibit the growth of many pathogens
What are the cells of the innate immune system?
Polymorphonuclear cells
- Neutrophils
- Eosinophils
- Basophils
- Monocytes and macrophages
- NK cells
- Dendritic cells
What are the soluble components of the innate immune system?
- Complement
- Acute phase proteins
- Cytokines and chemokines
What are the key features of the innate immune response?
Identical responses in all individuals Cells express receptors that allow them to detect and home to sites of infection Cells express genetically encoded receptors (PRRs) Phagocytic capacity to engulf pathogens Cells secrete cytokines and chemokines that regulate the immune response
Describe the role of neutrophils.
- Acute inflammation/Migrate rapidly to injury site
- Express receptors for cytokines and chemokines to detect inflammation
- Express Pattern Recognition Receptors to detect pathogens
- Express Fc receptors for immunoglobulin to detect immune complexes
- Perform phagocytosis and oxidative and non-oxidative killing
- Release enzymes, histamine, lipid mediators of inflammation from granules
- Secrete cytokines and chemokines to regulate inflammation
Describe the role of macrophages.
Present within tissue - Express receptors for cytokines and chemokines, express Fc receptors for immune complexes, express PRR - Perform phagocytosis and oxidative and non-oxidative killing - Secrete chemokines and cytokines to regulate inflammation - CAN process and present antigen to T cells
Describe phagocyte recruitment.
- Cellular damage and bacterial products trigger local production of inflammatory mediators (cytokines and chemokines)
- Cytokines activate vascular endothelium increasing permeability
- Chemokines attract phagocytes
How does the innate immune system recognise microorganisms?
Pattern recognition receptors (PRRs) - Toll-like receptors and mannose receptors recognise generic motifs known as pathogen-associated molecular patterns (PAMPs) e.g. bacterial sugars, DNA and RNA Fc receptors on innate immune cells allows them to bind to Fc portion of immunoglobulins to allow phagocytes to recognise immune complexes
Describe endocytosis.
- Opsonins act as a bridge between the pathogen and the phagocyte receptors
- Antibodies bind to Fc receptors on phagocytes
- Complement components can bind to complement receptors (e.g. CR1)
- Acute phase proteins (e.g. CRP) will also promote phagocytosis
What is a phagolysosome?
Phagolysosome = a phagosome fused with a lysosome
- forms a protected compartment in which killing of the organism occurs
Describe oxidative killing.
- NADPH oxidase converts oxygen into reactive oxygen species - superoxide and hydrogen peroxide
- Myeloperoxidase catalyses the production of hydrochlorous acid from hydrogen peroxide and chloride
- Hydrochlorous acid is a highly effective oxidant and anti-microbial
Describe non-oxidative killing.
Killing by release of bactericidal enzymes e.g. lactoferrin and lysozyme into the phagolysosome - The range of enzymes results in a broad range of cover against bacteria and fungi


Describe the role of natural killer cells.
Speacialised lymphocytes that are in the blood and may migrate to inflamed tissue
Express inhibitory receptors for self HLA class 1 which prevents inappropriate activation by normal self-cells
Express range of activatory receptors including natural cytotoxicity receptors that recognise heparan sulphate proteoglycans
Integrate signals from inhibitory and activatory receptors (usually inhibitory signals dominate)
Cytotoxic - kill ‘altered self’ cells (i.e. malignancy or virus-infected cells)
Secrete cytokines to regulate inflammation and promote dendritic cell function
Where are dendritic cells found?
Peripheral tissue
Describe the role of immature dendritic cells.
Detect inflammation - express receptors for cytokines and chemokines
Detect pathogens - express pathogen recognition receptors
Detect immune complexes - express Fc receptors for immunoglobulin
Capable of phagocytosis
What triggers dendritic cells to mature?
They do so following phagocytosis
Describe the role of mature dendritic cells.
Upregulate expression of HLA molecules
Express co-stimulatory molecules
Migrate via lymphatics to lymph nodes
Present processed antigen to T cells in lymph nodes to prime the adaptive immune system
Express cytokines to regulate the immune response


What are the components of the adaptive immune system?
Humoral Immunity - B lymphocytes and antibody
Cellular Immunity - T lymphocytes (CD4+ and CD8+)
Soluble Components - cytokines and chemokines
What are the 4 key features of the adaptive immune system?
- Wide repertoire of antigen receptors - VDJ recombination with mechanisms to delete or tolerate these cells
- Exquisite specificity
- Clonal expansion - cells with appropriate specificity will proliferate during infection
- Immunological memory
Define primary lymphoid organ and describe each one.
Organs involved in lymphocyte development
- Bone marrow - both T and B lymphocytes are derived from haematopoietic stem cells in BM but only B cells mature here
- Thymus - site of T cell maturation - most active in fetal and neonatal period, involutes after puberty
Define the secondary lymphoid organs and state what they are.
Anatomical sites of interaction between naïve lymphocytes and microorganisms in which immune reaction occurs
- Spleen
- Lymph nodes
- Mucosal associated lymphoid tissue (MALT)


Describe T lymphocyte maturation.
- Arise from haematopoietic stem cells
- Exported as immature cells to thymus where they undergo selection
- Mature T cells enter circulation and reside in secondary lymphoid organs
What do CD8 T cells recognise?
Peptides presented by HLA Class I
What do CD4 T cells recognise?
Peptides presented by HLA Class II
What selection process of T cells occurs?
Based on affinity to HLA molecules
- Low affinity = useless so removed
- High affinity = dangerous/autoimmune so removed
- 10% develop further
What is the role of CD4 T cells?
Recognise peptides derived from EC proteins
Provide help for development of full B cell response
Provide help for the development of some CD8+ T cell responses
Peptides usually presented on HLA Class 2
Immunoregulatory functions via cell: cell interactions and cytokine expression
- AKA T helper cells
What is the role of Th1 CD4 T cells?
Express CD4 and secrete IFN-gamma and IL2 to help CD8 and macrophages
What is the role of T follicular CD4 T cells?
Promote germinal centre reactions and differentiation of B cells into IgG and IgA secreting plasma cells
What is the role of regulatory CD4 T cells?
Express CD25 and transcription factor Foxp3
Secrete IL10 (immunosuppressive cytokine) to inhibit immune response
Express CTLA4 to directly inhibit T cell activation
What is the role of CD8 cytotoxic T cells?
Recognise peptides derived from intracellular proteins presented on HLA Class 1
Kills cells directly via perforin (pore-forming) and granzymes (expression of Fas ligand)
Secrete cytokines (e.g. IFN-gamma, TNF-alpha)
Important against viruses and tumours
Describe T cell memory.
Response to successive exposures of antigen
Pool of memory T cells ready to respond to antigen
More easily activated than naïve cells


What is the tolerance mechaniusm of B cells?
If they recognise self then they are negatively selected/deleted
What happens when B cells encounter antigens?
Early IgM Response - Cell differentiates into IgM secreting plasma cell
Germinal Centre Reaction - Somatic hypermutation and isotype switching from IgM to IgG/A/E
Describe the process of germinal centre reactions that develop an antibody response.
Dependent on T helper cells
- Dendritic cells prime CD4+ T helper cells
- CD4+ cells then help B cell differentiation (mediated by CD40L: CD40)
- B cells proliferate with the help of CD4 T cells
- Undergo somatic hypermutation and isotype switching (from IgM to IgG/A/E)
- Become plasma cells - produce antibodies
Describe the structure of an immunoglobulin.
Soluble proteins made up of 2 heavy chains + 2 light chains
Heavy chain determines antibody class (e.g. M, G, A)
Effector function determined by constant region (Fc) - heavy chain
Antigen binding region (Fab) recognise antigens - heavy and light chains
What are the functions of antibodies?
Identify pathogens and toxins (Fab-mediated)
Interact with other components of immune system to remove pathogens (Fc-mediated) - Complement, Phagocytes, NK cells
Particularly important in defence against BACTERIA
How do antibodies provide defence against infection?
Neutralisation - binds to receptors on target molecule so it cannot bind to anything else thereby blocking interaction with cells
Describe IgA antibodies.
Protection of mucosal surfaces via salivary, respiratory, gastrointestinal and lacrimal secretions = secretory function
- Dimer form with 4 binding sites found in mucosal and epithelial surfaces e.g. gut, mouth
- Monomer circulates in serum
IgA main antibody in breast milk, providing passive immunity in neonates
Describe IgG antibodies.
Most abundant antibody type
Monomor
Describe IgM antibodies.
Pentamer
Primary response against pathogens
Describe IgE antibodies.
Parasites and Type I hypersensitivity reactions
Describe B cell memory.
Decreased lag time between antigen exposure and antibody production (to 2-3 days)
Increased antibody titres produced
Response dominated by IgG antibodies of high affinity - compared to IgM in primary response
Response can be independent of help from CD4 cells



What is complement?
Over 20 tightly regulated linked proteins that are produced by liver
Inactive in the circulation
When activated by enzymes they activate other proteins in the biological cascade to produce a rapid, highly amplified response
What are the 3 pathways of complement activation?
Classical - C1, C2 and C4
Mannose Binding Lectin (MBL) - C2 and C4
Alternative
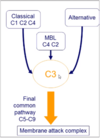
What is the final common pathway in complement activation?
All routes converge on C3
Final common pathway C5-C9 to membrane attack complex

Describe the classical pathway of complement activation.
Activated by immune complexes
- Formation of antibody-antigen immune complexes results in conformational change in antibody shape which exposes binding site for C1
- Binding of C1 to antibody results in cascade activation (dependent on the adaptive immune response so its not an early response)
Describe the Mannose Binding Lectin (MBL) pathway of complement activation.
MBL → C2, C4 → C3
- Binding of MBL to microbial cell surface CHO → Direct stimulation of classical pathway (only C4, C2)
- Not dependant on acquired immune response
Describe the alternative pathway of complement activation.
Directly triggered by binding of C3 to bacterial cell wall components (lipopolysaccharide of gram-negative bacteria)
Not dependent on adaptive immune response
Involves factors: B, I, P
Describe the common pathway of complement activation.
Convergence occurs at C3
- Activation of C3 convertase = major amplification step
- Triggers formation of membrane attack complex MAC via C5-9
- MAC makes holes in membranes hence killing pathogen
What are the roles of complement fragments released during complement activation?
Increase vascular permeability and cell trafficking to inflammation site
Opsonisation of immune complexes to keep them soluble
Opsonisation of pathogens to promote phagocytosis
Phagocyte activation
Promotes mast cells/basophil degranulation
Makes holes in bacterial membranes


What are cytokines?
Small protein messengers with immunomodulatory function
Autocrine and paracrine dependent action
- IL2, IL6, IL10, IL12, TNF-alpha, TGF-beta
What are chemokines?
Type of cytokines
Direct recruitment of leukocytes in inflammatory response via CCL19 and CCL21 = ligands for CCR7 (receptors)
Important in directing dendritic cell trafficking to lymph nodes
What are the causes of secondary immune deficiences?
- Malnutrition - most common globally
- Infections - HIV, Measles, TB, SARS-CoV-2
- Age extremities
- Splenic surgery/trauma
- Drugs - glucocorticoids (commonest), cytotoxic agents, chemotherapy, calcineurin inhibitors, antiepileptics, DMARDs, JAK inhibitors
- Genetic or Metabolic disease
- Haematological cancers
- Biological agents/cellular therapy - anti-CD20 etc or rituximab (antibody therapies)
What is Good’s syndrome?
- Thymoma and Antibody deficiency
- Combined T and B cell absence/defect
- CMV PJP and muco-cutaneous candida
- Increased risks of autoimmune disease
- Pure red cell aplasia, Myasthenia gravis, Lichen planus
What questions should you ask to evaluate secondary immune deficiency?
- Clinical history of infection
- Childhood illnesses/loss of schooling
- Other illness/PMH
- Family history of infection/autoimmune/cancers
- Medication history
- Vaccine history and any reactions
What investigation should be done for immune defects?
-
FISH for immunodeficiency - Picks up 85% of immune defects
- Full blood count
- Immunoglobulins
- Serum complement (C3 and C4 – serum complex disease, SLE, C1 inhibitor deficiency (a primary immune deficiency))
- HIV test
-
First Line Investigations
- Renal and liver profile
- Calcium and bone profile
- Total protein and albumin
- Urine protein/Cr ratio
- Serum protein electrophoresis (monoclonal proteins found in MM, WMG, NHL and MGUS)
- Serum free light chains (b-lymphoma)
-
Second Line Investigations
- Measure conc of vaccine antibodies - tetanus (protein antigen) and pneumovax (carbohydrate antigen of 23 types)
What does an isolated reduction in IgG suggest?
- Protein loosing enteropathy
- Prednisolone
What does a reduction in IgG and IgM suggest?
- B cell neoplasm
- Rituximab
What does a reduction in IgG and IgA suggest?
- Primary antibody deficiency
What is the management of secondary immune deficiencies?
- Treat underlying cause
- Advise measures to reduce exposure
- Immunisation against respiratory viruses and bacteria and offer vaccines to household contacts
- Education to treat bacterial infections promptly (use of rescue antibiotics)
- Prophylactic antibiotics for confirmed recurrent bacterial infections
- IgG replacement therapy – if other management have failed
Describe the structure of HIV?
- HIV has an icosahedral (20-faced) structure
- Double-stranded RNA
- Genome is diploid
- Contains 9 genes that encode 15 structural, regulatory and auxiliary proteins
- I.E. gp120, gp41 and RT
How does HIV replicate?
Inside a cell using Reverse Transcriptase to convert RNA to DNA which is integrated into the host genome
What is the target of HIV?
- CD4+ T-helper cells
- CD4+ monocytes
- CD4+ dendritic cells
What are the possible routes of transmission of HIV?
- Sexually
- Infected blood – transfusion, needle sharing, blood products
- Vertical (mother to child) – ante-/intra-partum, breastmilk
Describe the life cycle of HIV?
- Attachment and entry
- Reverse transcription and DNA synthesis
- Integration
- Viral transcription
- Viral protein synthesis
- Assembly of virus and release of virus
- Maturation

Describe the clinical course of HIV.

What are the immunological features of HIV infection?
- CD4 T cell depletion
- Chronic immune activation
- Impairment of CD4 and CD8 T cell function
- Disruption of lymph node architecture
- Impaired ability to generate protective T and B cell immune responses
- Loss of antigen-specific humoral immune responses
What are the mechanisms of HIV long-term non-progressors?
-
Host genetic factors:
- HLA profile - Heterozygosity for 32-bp deletion in chemokine-r CCR5
- MBL alleles - TNF c2 microsatellite alleles
- Gc vitamin D-binding factor alleles
-
Host immune response factors
- Effective CTL, HTL and humoral responses
- Secretion of:
- CD8 antiviral factor
- IL-16
- Secretion of chemokines that block HIV entry co-receptors CCR5 and CXCR4
- Maintenance of functional lymphoid tissue architecture
-
Virologic factors
- Infection with attenuated strains of HIV
How is HIV diagnosed and monitored?
- Detection via:
- Anti-HIV antibodies (ELISA) – screening test
- Anti-HIV antibodies (Western Blot) – confirmation test
- Viral load (viral RNA detection using PCR) – very sensitive; steps:
- CD4+ T Cell Counts – monitored using flow cytometry
- The onset of AIDS correlates with a decrease in the number of CD4+ T cells
- Antigens on T cells:
- CD3, CD4, CD8, CD19, CD56
- Antigens on T cells:
- The onset of AIDS correlates with a decrease in the number of CD4+ T cells
What is the best predictor of HIV prognosis?
Initial baseline plasma viral load
- Higher the load the sooner the progression to AIDS if not treated
At what CD4 T cell counts should preventative measure be taking against:
- PCP
- Toxoplasma gondii
- Mycobacterium avium complex (MAC)
- PCP = 200 x 109/L
- Toxoplasma gondii = 100 x 109/L
- Mycobacterium avium complex (MAC disease) = 75 x 109/L
How can resistance against anti-retroviral therapy be tested for?
- Phenotypic - viral replication is measured in cell cultures under selective pressure of increasing concentrations of antiretroviral drugs (compared to wildtype)
- Genotypic - mutations detected by sequencing amplified HIV genome
What is contained within HAART?
-
Combination of ≥3 ART drugs = Triple Therapy:
- 2 backbone drugs - x2 NRTIs
- ≥1 binding agent - x1 NNRTI or INI
Name some examples of each type of ART backbone?
- Nucleoside Reverse Transcriptase Inhibitors (NRTI) – e.g. Zidovudine/AZT, abacavir
- Nucleotide RTI – e.g. Tenofovir
- Non-NRTI (NNRTI) – e.g. Efavirenz
- Protease inhibitor (PI) – e.g. Indinavir
Name some examples of each type of ART binding agents?
- Integrase inhibitors (INI) – e.g. Raltegravir
- Attachment inhibitors (AI) – e.g. Maraviroc
- Fusion inhibitors (FI) – e.g. Enfuvirtide
What interactions exist with HAART?
- PI – block cytochrome P450
- Efavirenz – P450 inducer
- INI – interacts with indigestion remedies (Gaviscon, aluminium salts, calcium salts) and is sequestered which can be very bad as some INI is absorbed but very little and so resistance is bred very quickly
What are the limitations and complications of HAART?
- Adherence – most common reason for failure
- Does NOT eradicate latent HIV-1
- Fails to restore HIV-specific T cell responses
- Threat of drug resistance
- Significant toxicities
- High pill burden
- Quality of life
- Cost - >40% with no access
What methods work to reduce the spread of HIV?
- Male circumcision (APCs in foreskin at a high density)
- Condoms
- PrEP (Truvada)
- TasP (Treatment as Prevention)
What cures exist for HIV?
- Allogenic SCT from a CCRδ32 HLA matched donor
- CCRδ32 are unable to contract HIV – found in 1% of north-west European patients
- Shock and Kill

Describe B lymphocyte maturation.
- Initially exist in periphery as IgM B cells
- Undergo germinal centre reaction to differentiate into plasma cells expressing IgG, IgE and IgA
What are the most relevant proteins in terms of transplantation?
- ABO blood group
- HLA - MHC
- Other minor histocompatibility genes
What are the two major forms of rejection?
- T cell-mediated rejection
- Antibody-mediated rejection
Where are HLA expressed?
-
HLA Class I (A, B and C) = ALL cells
- Thought to be the most immunogenic
-
HLA Class II (DR, DQ, DP) = APCs
- Upregulated on other cells under stress
What are the features of HLA?
- They are highly polymorphic with hundreds of alleles for each locus
- High degree of variability from the areas of protein lining the peptide-binding groove which allows us to present a wide variety of antigens in that peptide-binding groove to the cells of the immune system
What HLA molecules are the most important to match in transplantation?
A, B and DR
- DR > B > A

Describe T-cell mediated rejection.
-
Phase 1 = Activation of T-cells
- Presentation of foreign HLA antigens in MHC by APCs and co-stimulatory signals activate T-cells within lymph nodes
- This leads to effector phase of rejection → inflammation caused leads to graft dysfunction
-
Phase 2 = Actions of activated T cells
- Proliferation
- Product cytokines (IL2 is important)
- Provide help to CD8+ cells
- Provide help for antibody production
- Recruit phagocytic cells
- Phase 3 = Effector Phase (Image)

What are the effects of cytotoxic T cells have on a transplanted organ?
- Granzyme B (toxin)
- Perforin (punch holes)
- Fas-ligand (apoptosis)
What are the effects of macrophages have on a transplanted organ?
- Phagocytosis
- Proteolytic enzymes
- Cytokine release
- O2 and N2 radicals
What are the histological features of T cell mediated rejection?
- Lymphocytic interstitial infiltration
- Ruptured tubular basement membrane
- Tubulitis - inflammatory cells within the tubular epithelium
- Macrophages

Describe antibody mediated rejection.
- Phase 1: recognition of foreign antigens
- Phase 2: activation of antigen-specific lymphocytes
-
Phase 3: effector phase - antibodies in graft
- Antibodies bind to antigens (HLA) on the endothelium of the blood vessels in the transplanted organ
- Antibodies activate complement
- Form MAC → endothelial cell lysis
- Recruit inflammatory cells to the microcirculation
- Antibodies crosslink the MHC molecules
- The antibodies can also directly recruit mononuclear cells, NK cells and neutrophils → capillaritis
- Phase 4: graft fibrosis
What are the cardinal features of antibody-mediated rejection?
Capillaritis = inflammatory cells in capillaries of the kidney → injury
What is the histology of antibody-mediated rejection?
- Inflammatory cell infiltrate
- Capillaritis
- Fixation of complement fragments on endothelial cell surfaces
What kind of damage is caused by T-cell and antibody medtaited rejection?
- T-cells = Interstitial damage
- Antibodies = Endothelial damage
How is graft rejection prevented?
- HLA typing
- Screening for anti-HLA antibodies
Describe anti-HLA antibody screening.
-
Cytotoxicity assays
- Inspects if recipient’s serum will kill the lymphocytes of the donor
- Positive crossmatch suggests that there is cell lysis
-
Flow cytometry
- Inspects if recipient’s serum binds to the donor’s lymphocytes using fluorescent anti-human immunoglobulin
-
Solid phase assays
- Recipient’s serum is mixed with beads and fluorescently labelled immunoglobulin is used to determine which HLA epitopes the antibodies bind to
- Patients that have antibodies to lots of different types of antibodies are regarded as highly sensitised
How can organ mismatch issues be overcome?
- Improve transplantation across tissue barriers
- More donors
- Organ exchange programmes
- Future: xenotransplantation (animals), stem cell research
What are the 3 signals to activate T-cells?
- APC MHC to T-cell TCR = Main signal
- APC CD80/CD86 to T-cell CD28 - CD80/86 to CTLA4 suppresses immune reactions
- Cytokine IL-2 to T-cell CD25 - after T-cell activation, autocrine IL-2 is released to further activate
What immunosuppressants are used in the management/prevention of T cell mediated rejection?
- Steroids - prevent general T-cell mediated rejection
- Inhibitors of cell signalling - Calcineurin inhibitors
- Anti-proliferative agents
-
Inhibitors of cell surface receptors
- Alemtuzumab = anti-CD52 monoclonal antibody that causes lysis of T cells
- Basiliximab = anti-CD25 monoclonal antibody which targets the IL-2-R à less proliferation
What are the main targets for immunosuppression for antibody-mediated rejection?
- B-cell activation
- Plasma cell secretion of antibodies
- Antibody effects on endothelium
What immunosuppressants are used in the management/prevention of antibody-mediated rejection?
- Rituximab - B cell depletion
- BAFF inhibitors
- Proteasome inhibitors - block production of antibodies by plasma cells
- Complement inhibitors - block complement binding to endothelial cells
What is the modern transplant immunosuppression regimen?
- Induction agent = OXT3/ATG, anti-CD52, anti-CD25
- Given at time of transplantation or just before to prepare the patient to receive the foreign organ
- Baseline immunosuppression = calcineurin inhibitor + mycophenolate mofetil / azathioprine ± steroids
What is the management of acute transplant rejection?
- Cellular = Steroids, OKT3, ATG
- Antibody-mediated = IVIG, Plasmapheresis, Anti-C5, Anti-CD20
Describe post-transplant malignancy.
- Viral-associated malignancies are much more common
- Kaposi sarcoma (HHV8)
- Lymphoproliferative disease (EBV)
- Skin cancer is 20x more common
- Risk of other cancers is also increased
What are the features of immunodeficiency?
- Infection
- Two major or one major and recurrent minor infections in one year
- Atypical organisms
- Unusual sites
- Poor response to treatment
What are the features of primary immune deficiency?
- Infection
- Family history
- Young age at presentation
- Failure to thrive
What are the common investigations for primary immune deficiency?
- White cells
- Full blood count
- Lymphocyte subsets
- White cell migration/function
- Immunoglobulins
- IgM, IgG, IgA
- Specific Igs and response to vaccination
- Complement
- Complement function
- Individual complement components
What is the role of phagocytes?
- Express cytokine/chemokine receptors to locate sites of infection
- Express genetically encoded receptors for detection of pathogens at site of infection
- Pattern recognition receptors which recognise generic motifs known as pathogen-associated molecular patterns (PAMPs) such as bacterial sugars, DNA, RNA
- Express Fc receptors for detection of immune complexes
- Phagocytic capacity to engulf the pathogens
- Secrete cytokines and chemokines to regulate immune response
What are the types of primary phagocyte deficiency?
- Failure to produce neutrophils
- Failure of stem cells to differentiate along myeloid or lymphoid lineage
- Reticular dysgenesis = Autosomal recessive severe SCID
- Specific failure of neutrophil maturation
- Kostmann syndrome
- Cyclic neutropenia
- Failure of stem cells to differentiate along myeloid or lymphoid lineage
- Defect of phagocyte migration
- Leukocyte adhesion deficiency
- Failure of oxidative killing mechanisms
- Chronic granulomatous disease
- Cytokine deficiency
- IL12, IL12R, IFNg or IFNg R deficiency
What is Reticular dysgenesis?
- Autosomal recessive severe SCID
- Mutation in mitochondrial energy metabolism enzyme adenylate kinase 2 (AK2)
What is Kostmann syndrome?
- Autosomal recessive severe congenital neutropenia
- Classical form due to mutation in HCLS1-associated protein X-1 (HAX1)
What is Cyclic neutropenia?
- Autosomal dominant episodic neutropenia every 4-6 weeks
- Mutation in neutrophil elastase (ELA-2)
What is Leukocyte adhesion deficiency?
- Deficiency of CD18 (b2 integrin subunit)
- CD11a/CD18 (LFA-1) is expressed on neutrophils
- Binds to ligand (ICAM-1) on endothelial cells and so regulates neutrophil adhesion/transmigration
- In Leukocyte adhesion deficiency the neutrophils lack these adhesion molecules and fail to exit from the bloodstream
- Very high neutrophil counts in blood
- Absence of pus formation
- CD11a/CD18 (LFA-1) is expressed on neutrophils
What are the features of leukocyte adhesion deficiency?
- Very high neutrophil counts in blood
- Absence of pus formation
What are the features of chronic granulomatous disease?
- Absent respiratory burst
- Excessive inflammation
- Granuloma formation
- Lymphadenopathy
- Hepatosplenomegaly
What is chronic granulomatous disease?
- Deficiency of one of components of NADPH oxidase
- Inability to generate oxygen free radicals results in impaired killing
- Persistent neutrophil/macrophage accumulation
- Failure to degrade antigens
What are the appropriate investigations for suspected chronic granulomatous disease?
-
Nitroblue tetrazolium (NBT) test
- NBT is a dye that changes colour from yellow to blue, following interaction with hydrogen peroxide
-
Dihydrorhodamine (DHR) flow cytometry test
- DHR is oxidised to rhodamine which is strongly fluorescent, following interaction with hydrogen peroxide
What infections are common in patients with phagocyte deficiency?
- Bacterial infections
- Staphylococcus aureus
- Enteric bacteria
- Fungal infections
- Candida albicans
- Aspergillus fumigatus and flavus
- Mycobacterial infection
- Mycobacterium tuberculosis
- Atypical Mycobacteria
What is the management of phagocyte deficiency?
- Aggressive management of infection
- Infection prophylaxis
- Antibiotics → e.g. Septrin
- Anti-fungals → e.g. Itraconazole
- Oral/intravenous antibiotics as needed
- Infection prophylaxis
- Definitive therapy
- Haematopoietic stem cell transplantation
- Specific treatment for CGD → Interferon gamma therapy
What are the types of primary natural killer cells deficiency?
-
Classical NK deficiency
- Absence of NK cells within peripheral blood
- Abnormalities described in GATA2 or MCM4 genes in subtypes 1 and 2
-
Functional NK deficiency
- NK cells present but function is abnormal
- Abnormality described in FCGR3A gene in subtype 1
What infections are common in primary natural killer cells deficiency?
- Virus infection
- Herpes virus infection
- Herpes Simplex Virus I and II
- Varicella Zoster Virus
- Epstein Barr Virus
- Cytomegalovirus
- Papillomavirus infection → associated cancers
- Herpes virus infection
What infections are common in patients with natural killer cells deficiency?
- No good trial data
- Prophylactic antiviral drugs such as acyclovir or gancyclovir
- Cytokines such as IFN-alpha to stimulate NK cytotoxic function
- Haematopoietic stem cell transplantation in severe phenotypes
What are the types of primary deficiency of complement?
- Complement deficiency
- Classical pathway deficiency (C2, C1q)
- MBL deficiency MBL2 mutations are common but not usually associated with immunodeficiency
What is Complement deficiency?
- Susceptibility to bacterial infections → especially encapsulated bacteria
- Neisseria meningitides → properidin and C5-9 deficiency
- Haemophilus influenzae
- Streptococcus pneumoniae
What is Classical pathway deficiency?
- Deficiency in C2 and C1q
- Susceptibility to SLE
- Failure of phagocytosis of dead cells
- Increased nuclear debris
- Failure to clear immune complexes
- Immune complex deposition in blood vessels
How can SLE lead to to functional complement deficiency?
- Active lupus causes persistent production of immune complexes
- Consumption of complement leading to functional complement deficiency
How can nephritic factors lead to functional complement deficiency?
- Nephritic factors are auto-antibodies directed against components of the complement pathway
- Nephritic factors stabilise C3 convertases resulting in C3 activation and consumption
- Often associated with glomerulonephritis (classically membranoproliferative)
- May be associated with partial lipodystrophy
What are the appropriate investigation for deficiency in complement?
- Measure C3 and C4
- Functional
- CH50 - classical pathway
- AP50 - alternative pathway

What is the management of complement deficiences?
- Vaccination
- Boost protection mediated by other arms of the immune system
- Meningovax, Pneumovax and HIB vaccines
- Prophylactic antibiotics
- Treat infection aggressively
- Screening of family members
What is the pathophysiology of SCID?
- Defects of Haemopoetic stem cells
- Reticular dysgenesis - most severe SCID
- Defects of Lymphoid precursors
- Less severe forms of SCID
Describe X-linked SCID.
- 45% of all SCID
- Mutation of common gamma chain on chromosome Xq13.1
- Shared by cytokine receptors for IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21
- Inability to respond to cytokines causes early arrest of T cell and NK cell development and production of immature B cells
- Phenotype
- Very low or absent T cell numbers
- Very low or absent NK cell numbers
- Normal or increased B cell numbers but low Igs
Describe ADA deficiency.
- 16.5% of all SCID
- Adenosine Deaminase Deficiency
- Enzyme required for cell metabolism in lymphocytes
- Phenotype
- Very low or absent T cell numbers
- Very low or absent B cell numbers
- Very low or absent NK cell numbers
What protects the SCID neonate in their first 3 months of life?
Source of circulating IgG in the neonate

What are the signs of SCID?
- Unwell by 3 months of age
- Infections of all types
- Failure to thrive
- Persistent diarrhoea
- Unusual skin disease
- Colonisation of infant’s empty bone marrow by maternal lymphocytes
- Graft versus host disease
- Family history of early infant death
Describe T cell selection and central tolerance.

Describe CD8+ T celss.
Specialised cytotoxic cells
- Recognise peptides derived from intracellular proteins in association with HLA class I
- HLA-A, HLA-B, HLA-C
- Kill cells directly
- Perforin (pore forming) and granzymes
- Expression of Fas ligand
- Secrete cytokines
- Particularly important in defence against viral infections and tumours
Describe CD4+ cells.
Specialised helper cells
- Recognise
- Peptides derived from extracellular proteins
- Presented on HLA Class II molecules (HLA-DR, HLA-DP HLA-DQ)
- Immunoregulatory functions via cell:cell interactions and expression of cytokines
- Provide help for development of full B cell response
- Provide help for development of some CD8+ T cell responses
What are the CD4+ T cell subsets?

What is DiGeorge syndrome?
- Deletion at 22q11.2 → TBX1 may be responsible for some features
- Usually sporadic rather than inherited
- Normal numbers B cells
- Reduced numbers T cells
- Homeostatic proliferation with age
- Immune function usually only mildly impaired and improves with age
What are the signs of DiGeorge syndrome?
- High forehead
- Developmental defects in the pharyngeal pouch
- Low set
- Abnormally folded ear
- Cleft palate
- Small mouth and jaw
- Hypocalcaemia
- Oesophaegeal atresia
- Underdeveloped thymus
- Complex congenital heart disease
What is the Bare lymphocyte syndrome - type 2?
- Defect in one of the regulatory proteins involved in Class II gene expression
- Regulatory factor X
- Class II transactivator
- Absent expression of MHC Class II molecules
- Profound deficiency of CD4+ cells
- Usually have normal number of CD8+ cells
- Normal number of B cells
- Low IgG or IgA antibody due to lack of CD4+ T cell help
BLS type 1 also exists due to failure of expression of HLA class I
What are the signs of bare lymphocyte syndrome?
- Unwell by 3 months of age
- Infections of all types
- Failure to thrive
- Family history of early infant death
What are the clinical features of lymphocyte deficincies?
- T cell deficiency
- Viral infections
- Cytomegalovirus
- Fungal infection
- Pneumocystis
- Cryptosporidium
- Some bacterial infections
- Especially intracellular organisms
- Mycobacteria tuberculosis
- Salmonella
- Early malignancy
- Viral infections
What are the appropriate investigations for suspected T cell deficiences?
- Total white cell count and differential
- Lymphocyte subsets
- Quantify CD8 T cells, CD4 T cells as well as B cells and NK cells
- Immunoglobulins
- If CD4 T cell deficient
- Functional tests of T cell activation and proliferation
- HIV test
What are the results of investigations for T cell deficiencies?

What is the management of T cell immunodeficiency?
- Aggressive prophylaxis/treatment of infection
-
Haematopoieitic stem cell transplantation
- Replace abnormal populations in SCID
- Replace abnormal cells - class II deficient APCs in BLS
-
Enzyme replacement therapy
- PEG-ADA for ADA SCID
- Gene therapy
-
Thymic transplantation
- Promote T cell differentiation in Di George syndrome
- Cultured donor thymic tissue transplanted to quadriceps muscle
Describe B cell central tolerance.

What are the types of primary T cell immunodeficiency?
- SCID
- DiGeorge
- BLS
Describe immunoglobulins?
- Soluble proteins made up of two heavy and two light chains
- Heavy chain determines the antibody class
- IgM, IgG, IgA, IgE, IgD
- Antigen is recognised by the antigen binding regions (Fab) of both heavy and light chains
- Effector function is determined by the constant region of the heavy chain (Fc)
- Heavy chain determines the antibody class

What is the function of antibodies?
- Identification of pathogens and toxins (Fab mediated)
- Interact with other components of immune response to remove pathogens (Fc mediated)
- Complement
- Phagocytes
- Natural killer cells
- Particularly important in defence against bacteria of all kinds


















