Homework Problems Flashcards
(14 cards)
A magnetic system has specific heat which is a function of applied magnetic field, H and temperature, T.
It can be represented theoretically by
C(T,H) = C(H/T) = N*(H/T)2 / cosh2(H/T) where N is constant.
(a) Expand C(T,H) in power of (H/T) up to (H/T)4 assuming H << T.
(b) Sketch (freehand) the behavior of your answer as a function of x = H/T.

A system contains n atoms, each of which can only have zero or one quanta of energy. How many ways can arrange r quanta of energy when n = 2x1023; r = 10 23?
(How can you arrange the amount of quanta of energy r to the amount of atoms n.)
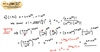
The probability P(n) that an event characterized by a probability p occurs n times in N trials is given by the binomial distribution
P(n) = [N! / n!(N-n)!] pn (1 - p)N-n.
Consider a case where p << 1 and N >> 1.
(a) Show that (1 - p)N-n ≈ e-Np using ln(1-p) ≈ -p.
(b) Show that N!/(N-n)! ≈ Nn .
(c) Therefore, you can show that P(n) = (λn / n!) e-λ where λ = Np. You have just demonstrated that the binomial distribution for small p and large N turns into the Poisson distribution!

Two bodies, with heat capacities C1 and C2 (assumed independent of temperature) and initial temperatures T1 and T2 respectively, are placed in thermal contact. Show that their final temperature Tf is given by
Tf = (C1T1+C2T2)/(C1+C2).
If C1 is much larger than C2, show that
Tf ≈ T1+C2(T2−T1)/C1.

(3.3) Blundell & Blundell

If θ is a continuous random variable which is uniformly distributed between 0 and π, write down anexpression for P(θ). Hence find the value of the following averages:
(d) θn (for the case n ≥ 0)
(g) |cosθ|

Find the average energy E for
(a) An n-state system, in which a given state can have energy 0, 2ε, 3ε, …., nε.
(b) A harmonic osillator, in which a given state can have energy 0, 2ε, 3ε, …., (i.e., with no upper limit).

The key to this question is the recongization of the partition function being a geometric series.
Find the mass of a hydrogen molecule, given that the atomic weight of one hydrogen atom is 1.01 amu.
In reality, the hydrogen molecule is diatomic.
1.01 amu = 1.01 grams/mole = .001 kg/mole
so
(.001 kg/mole) / NA ≈ 3.3 x 10-27 kg.
Derive an explaination of the difference between a vertical spring and a horizontal spring.
Use energy conservation to show that you can treat a vertical spring as a horizontal spring. Hooke’s Law can be applied at the equilibrium position when mg = kxo and we do not need to consider the natural length of the spring to be our starting point as in the horizontal case.

A. x = mg/k
B. Classical view for a non equilibrium position: ½k(x-xo)2
Translational time invariance states PE = KE, since the only force is conservative.
So in terms of statistics then we take the average of x and the equilibrium point xo labled as (x-xo) which is named as a fluctuation/deviation.
½k(x-xo)2 = ½KBT = Eparticles
⇒ (x-xo) = (KBT/k)½.
C. m = (KBkT)½/g


A) What is the volume occupied by 1 mole of gas at 10-10 torr, the pressure inside an “ultra high vacuum” (UHV) chamber?
B) Calculate the average distance between the molecules.
Assuming T ≈ 300K,
V ≈ 1.875 x 1011 m3 (a macroscopic quanity)
n = 1/V (Proportions and ratios)
r ≈ 4.20 x 10-5 m.
A diffuse cloud of neutral hydrogen atoms (known as HI) in space has a temperature of 50K and number density 500 cm-3. Calculate the pressure (in Pa) and the volume (in cubic light yrs) occupied by the cloud if its mass is 100Msun.
Msun ≈ 1.99 x 1030 kg
1 light yr. ≈ 9.460 x 1015 m


For B) you know the average energy to be 3 · ½kBT, Ch. 5. To find the average energy of those hitting the wall, use the proper integral dervived in Ch. 6.



