Glycogen metabolism, glycolysis Flashcards
In what state is glucagon expressed? By what general mechanism does it act?
In the unfed state.
-acts by increasing cAMP in liver and adipose to make energy (catabolism) and decrease glycolysis.
In what state is epinephrine expressed? By what general mechanism does it act?
Fight or flight
-acts by increasing cAMP and calcium intake in muscle, adipose, and liver (muscle contraction!) to increase energy production by catabolism, including glycolysis
In what state is insulin expressed? By what general mechanism does it act?
Fed state
-increases tyrosine kinase activation and PIP3 in muscle, adipose, and liver, as well as inhibition of cAMP to increase uptake of glucose and glycogen synthesis while preventing energy formation.
In general, how is metabolism coordinated?
Hormone signaling
What is the insulin agonist?
epinephrin or glucagon
What receptor does epinephrine and glucagon bind to? What is the effect?
Glucagon and epinephrine can bind to GPCR. Effect is activation of adenylate cyclase –> cAMP –> protein kinase A –> kinase activity. Epinephrine can also elicit IP3 formation from PIP3 using phospholipase C, activated by G subunit alpha.
What effects do epinphrine and insulin have on PIP3?
Epinephrine cleaves it to form IP3, insulin phosphorylates PIP2 to form PIP3.
Which GLUT transporter is insuline dependent?
GLUT4 in muscle and adipose. GLUT4 transporter is endocytosed into the cytoplasm in the absence of insulin, and added back to the membrane when insulin is present.
Describe the Km values for the GLUT transporters, and how they relate to normal circulating glucose concentrations.
The normal circulating glucose level is 5mM. 3 of the GLUT transporters have Km values around that. These transporters rely mostly on glucose. GLUT2 in the liver, however, has a much higher Km, allowing glucose uptake only after a meal so that at normal or unfed conditions, it can send glucose off for other tissues such as the brain to use.
Describe an experiment supporting the insulin dependence of GLUT4.
A GLUT4 protein is MYC-tagged in the extracellular space, and EGFP-tagged on the cytoplasm side. We see basal GFP in the absence of insulin, and no MYC antibody is detected. When insulin is added, the MYC antibody signal overlaps with the GFP, showing the protein has moved to the cell membrane.
If glucose transport into and out of the cell is by diffusion, how do we regulate glucose in the cell?
It is phosphorylated at carbon 6 by one of two isoforms. Glucokinase in the liver, or hexokinase everywhere else. Glucose-6-P cannot pass through the membrane like glucose.
Describe the differences between glucokinase and hexokinase.
Glucokinase (liver) has a high Km for glucose, so it does not grab glucose as readily as hexokinase. Hexokinase (all other tissues) has a low Km for glucose, so it phosphorylates it more readily. Hexokinase is also inhibited by its product, glucose-6-P.
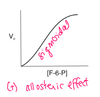
Describe the kinetics of glucokinase.
It is sigmoidal, suggesting allostery. The inflection point is around normal glucose levels so that glucokinase can be more sensitive. If glucose decreases a little, glucokinase activity decreases a lot. If glucose inreases a little, glucokinase activity increases a lot.
Describe how the monomeric protein glucokinase can be regulated by allostery.
If glucose binds, it falls into a closed conformation and becomes active. When ATP binds it is locked in. When glucose is not plentiful, glucokinase falls into an open and inactive conformation, which is slow to getting back to its open form.
Which types of tissues are glucose-dependent?
RBC, brain, renal medulla (i.e. these use only glucose for energy production). These tissues need glucose whether is it available or not, so glucose transport is insulin-independent.
Which tissues are glucose-independent?
Muscle, heart, adipose (though glucose transport is insulin-dependent). These tissues can store glucose.
Which tissues are glucose-producing?
Liver. Production is insulin-independent
Glucose phosphorylation by hexokinase is controlled by what type of regulation?
allosteric regulation by the product
Glucose phosphorylation by glucokinase is controlled by what type of regulation?
Substrate concentration.
What different pathways can glucose-6-P follow?
- glycolysis
- hexose monophosphate pathway
- glycogen formation
What types of bonds are found in glycogen?
- between linear glucose monomers: alpha 1,4 linkage
- between branch point: alpha 1,6 linkage
Why is excess glucose not just stored as glucose?
Each individual glucose monomer draws in water by osmosis. Osmosis looks at the number of individual molecules, not the size of each molecule, so by forming a single molecule of glycogen (albeit a large molecule), it can avoid osmotic pressure.
What is the function of glycogen in the liver versus in the muscle?
Liver: maintain blood glucose levels for all tissues
Muscle: fuel reserve for its own ATP generation
What are the three sources of glucose?
diet - immediate
glycogen - hours
gluconeogenesis - days
Which tissue has the ability to dephosphorylate glucose-6-P with a phosphatase?
The liver, so it can mobilize glucose to other tissues in need.
Describe the process of glycogen synthesis.
- glucose-6-P is isomerized by phosphoglucomutase to glucose-1-P
- glucose-1-P is activated by UDP-glucose phosphorylase using UTP to produce UDP-glucose and pyrophospate (PPi). PPi is hydrolyzed to inorganic phosphate, which drives the reaction forward.
- nascent glycogen chain is primed, as glycogen synthase cannot start from scratch, by UDP-glucose onto glycogenin, leaving behind UDP. UDP-glucose can be added to this by glycogen synthase. Thus, at the core of every glycogen granule is a glycogenin molecule.
- Glycogen is branched by the branching enzyme, by taking a chunk from the linear chain and moving it to a branching point.
Why is glycogen branched?
Phosphorylase chews away at glycogen from the end of the chain one molecule at a time. Thus, if there are more branches, then glucose can be mobilized more quickly if and when it is needed.
Why do plants store glucose in amylose in a linear chain witout any branches?
It does not need to rapidly mobilize glucose as we do.
Describe the breakdown of glycogen.
- glycogen phosphorylase shortens linear chains one molecule at a time by inserting a phosphate between bonds, forming glucose-1-P. (saves energy so we don’t have to re-phosphorylate glucose for use in glycolysis)
- phosphoglucomutase converts glucose-1-P to glucose-6-P for use in glycolysis or hexose pathway.
- debranching enzyme cuts off most of the branch and adds to the end of a linear chain for degradation by glycogen phosphorylase, and then a single glucose left behind is hydrolyzed to produce glucose (not glucose-1-P!)
How does glycogen phosphorylase overcome the competition of hydrolysis to break glycogen chains?
It holds glycogen very tightly so as to exclude water.
How much glucose is produced per breakdown of a glycogen branch?
1 molecule of glucose per branchpoint.
Describe the energetics of glycogen synthesis.
- energy input to activate glucose-1-P with UDP
- ATP to regenerate UTP
Total: 1 ATP molecule used.
Describe the energetics of glycogen breakdown.
- input of one inorganic phosphate per glycosidic bond broken via phosphorolysis
- one ATP molecule needed for every removal of a glucose at a branch point