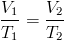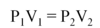Gases, Liquids, and Solids Flashcards
From the behavior of ideal gas systems to the properties of boiling liquids, use these cards to master the topic of the chemical phases of matter as tested in most introductory undergrad chemistry courses and even on the AP Chemistry exam. (48 cards)
Define and give the three examples of:
a phase
A phase is a physically distinct form of a substance that can be separated from another form. Generally phase is used interchangeably with “state of matter”.
Solid, liquid, and gas are the three phases of matter that most chemistry courses, as well as the AP Chemistry exam, will test.
Which two state functions can be used to actively change the phase of a substance?
Temperature (Heat, Enthalpy) can be added or removed. In general, an increase in temperature drives the phase change solid⇒liquid⇒gas.
Pressure (Volume) can be added or removed. In general, an increase in Pressure (or decrease in Volume) drives the phase change gas⇒liquid⇒solid.
What name is associated with a phase change from
- solid to liquid?
- liquid to solid?
- solid⇒liquid is fusion (melting)
- liquid⇒solid is freezing (solidification)
What name is associated with a phase change from
- gas to liquid?
- liquid to gas?
- gas⇒liquid is condensation
- liquid⇒gas is vaporization (boiling)
What name is associated with a phase change from
- gas to solid?
- solid to gas?
- gas⇒solid is deposition
- solid⇒gas is sublimation
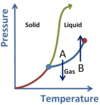
What phase is the substance in sections A, B, and C in the below diagram?

- A represents the Solid phase region.
- B represents the Liquid phase region.
- C represents the Gas phase region.

What phase conversions are being shown with arrows A and B?

- A is Fusion (or Melting)
- B is Freezing (or Solidification)

What phase conversions are being shown with arrows A and B?
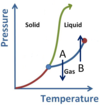
- A is Vaporization (or boiling)
- B is Condensation
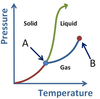
What phase conversions are being shown with arrows A and B?

- A is Sublimation
- B is Deposition

What points are arrows A and B pointing to?
- A is the Triple Point.
- B is the Critical Point.
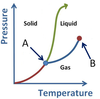
Note: though the concept of Plasma exists, the AP Chem exam does not explicitly test this as a phase.
Explain the significance of the critical point.
The critical temperature and critical pressure combine to be the critical point:
- the critical temperature is the temperature above which a distinct liquid-to-gas vaporization can no longer be accurately determined.
- the critical pressure is the pressure above which a distinct gas-to-liquid condensation can no longer be accurately determined.
Explain the significance of the triple point.
The triple point is the point at which a substance can exist in equilibrium in all three states (solid, liquid, and gas).
This means that instantaneously, a substance at its triple point can interconvert between any phase.
Ex: water at its triple point would exist as ice, liquid water, and steam - all at one temperature and pressure.
How does the phase diagram of water differ from the one below?

Water has a slightly negative sloped Solid/Liquid boundary.

This is due to water’s Solid phase being less dense than its Liquid phase.
Different materials require different applications of heat in order to transition into a new phase. Why is this?
The difference is due to intermolecular forces.
The attraction of molecules to each other determines at which temperature mixtures will have phase changes, and subsequently the amount of heat necessary to make that transition.
What types of intermolecular forces exist?
From strongest (1) to weakest (4), they are:
- Ionic Forces
- Hydrogen Bonds
- Dipole-Dipole
- London Dispersion Forces
Define:
dipole forces
Dipole forces occur when a molecule with polar bonds has the geometry to become overall polar. This results in partially positive and negative charges, and these opposite charges attract other charged molecules in the mixture.
Define:
hydrogen bonds
Hydrogen bonds are a product of H being covalently bonded to either O, N, or F. This is an extreme form of the dipole-dipole force.
The high electronegativity difference between these atoms and hydrogen creates strong dipoles, which consequently result in the strongest dipole-dipole interactions between molecules.
Define:
London Dispersion forces
Dispersion forces (also called Van der Waals forces) are an instantaneous polarization of molecules that would otherwise be non-polar.
This process is also referred to as an induced dipole because there is an instantaneous reorganization of the electron cloud leading to partial polarization. The more valence electrons present in either a molecule or in a mixture, the more possible repulsion of electron clouds, and the more induced dipoles.
Define:
ionic forces
Ionic forces occur when two oppositely charged ions (cations and anions) attract each other.
In general, ions attract to a polar molecule first, then attract each other - hence this is often called Ion-Dipole force. (Two Ions attracting each other would otherwise prefer to Ionic Bond, which is an intramolecular force, not an intermolecular force.)
Describe how the boiling point of a substance changes depending on the strength of the intermolecular forces present?
The stronger the intermolecular forces present, the higher the boiling point. This is due to the molecules being more tightly attracted to each other is the liquid phase.
In order of highest (1) to lowest (4) boiling points:
Ionic Forces
Hydrogen Bonds
Dipole-Dipole
Dispersion Forces
List forces from strongest to weakest:
Dipole-Dipole
Hydrogen Bonds
London Dispersion Forces
Ionic Forces
- Ionic Forces
- Hydrogen Bonds
- Dipole-Dipole
- London Dispersion Forces
Define:
Hv
Hv is the heat of vaporization. It represents the energy necessary to convert a liquid to a gas.
Define:
Hf
Hf stands for the heat of fusion. This is the amount of energy that is necessary to convert a solid to a liquid.
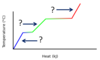
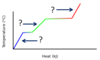
What processes are associated with the plateaus A and B, for the general heating curve below?

- At A, fusion is occurring.
Hf is used to calculate the heat needed for plateau A. - At B, vaporization is occurring.
Hv is used to calculate the heat needed for plateau B.