familial colon cancer - model of carcinogenesis - lecture notes - julia Flashcards
what does normal colon look like histologically?
- straight tubular structures with no lateral branching and a structured pattern of epithelial growth, differentiation and death
- tubular structures lined by cells
- some inflammatory cells around (not abnormal)
- number of crypts should be uniform
- cells turn over every 3-4 days

what does tubular adenoma look like histologically?
- benign colon polyp
- lack of organization to crypts

what does dysplasia look like histologically?
- cells crowded and there’s some loss of cell polarity with nuceli present at the cell surface
- loss of cytoplasmic differentiation (reduced mucin production)
- nuclei are enlarged and the chromatin is dense and irregularly distributed
- abnormal mitotic figures
- colonic mucosa no longer a single layer - stratified

what does adenocarcinoma look like histologically?
- invading into muscularis propria

what causes FAP? how does it relate to sporadic colon cancer?
- genetic defect in adenomatous polyposis coli gene - has regulatory roles in colonic cells
- over 80% of sporadic polyps share the genetic abnormality seen in patients with FAP
what causes hereditary non-polyposis colonic cancer (HNPCC)?
- familial form of colon cancer
- due to defects in DNA mismatch repair (MMR)
- results in accelerated tumor progression - accelerated growth and increased mutational events
what does APC do?
- regulates transcription and cell proliferation through its regulation of beta catenin
- in abscense of Wnt ligand, beta-catenin is sequestered in a multiprotein degradation complex of axin, APC, CKI and GSK3beta
- upon phosphorylation, beta catenin is ubiquitinated by b-TrCP-E3 ligase complex
- B-catenin is degraded and there’s no transcription of Wnt genes
- so APC involved in squestering beta catenin and therefore preventing wnt target genes from being transcribed
- when wnt ligand binds, beta catenin released, activates TCF which results in wnt target genes being activated
what does APC regulate? (4)
- the amount of beta catenin - free beta catenin in the nucleus can initiate cell division
- APC regulates microtubule assembly and has a role in cytoskeletal maintenance
- APC regulates AXIN independently of beta-catenin
- plays regulatory role in certain apoptosis pathways
what is the role of beta catenin?
- acts at progression of G1 and S into cell cycle
- abscense of beta catenin in the process ultimately favors apoptosis
- anchors cadherin to actin at adherens cell junctions - attach cells to each other and basement membrane
- promotion of beta catenin promotes adherence and cell to cell connections
where is beta catenin located in normal colonic cells? in cancerous or abnormal cells?
- normally has membranous localization
- most part of cell junctions
- in polyp cells, gets more localized to the cytoplasm and nucleus - implies more wnt signaling
- also free in cytoplasm in stem cells

how is mutated APC in colon cancer functionally different from normal APC?
- mutated = 1 allele has germ line trunctaion mutation
- trunctated => protein that can’t bind to beta catenin - allows free beta catenin to accumulate in cells - beta catenin can’t bind actin => increased cell proliferation
- may also not be able to bind microtubules => effected cytoskeletal and mitotic spindle cell functions
what is the normal process of crypt development?
- crypt ordered so that cell proliferation occurs toward the base of the crypt
- cells migrate toward the surface and differentiate
- eventually die and are shed into the lumen
- occurs continously over 3-4 days
what is the role of wnt and notch in development of colonic crypts?
- together may play role in maintaining stem cell compartment
- changes in notch associated with differentiating cells in transit amplifying compartments
- differentiated cells lose wnt activation
- processes have strict spacial relationships in the crypt
what are the consequences of mutating APC? (5)
- altered interactions with microtubles/F-actin => decreased cell migration => inappropriate accumulation of cells
- altered interactions with beta-catenin => decreased differentiation => maintains stem cell or transit amplifying cell character
- changes interactions with beta-catenin and microtubules => changes proliferation levels => maintains stem cell or transit amplifying cell character, but faulty and less efficient mitosis
- changes in interactions with microtubules => decreased genetic stability => chromosomal aberrations
- changes in interactions with microtubules and beta catenin => decreased apoptosis => inappropriate survival of damaged cells
what does normal crypt fission look like histologically?
- crypts normally form through an orderly process of crypt fission
- crypt numbers are normally held fairly constant
- indented region on right = almost completed crypt fission
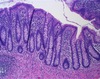
how does mutated APC affect crypt fission? what will it look like histologically?
- increased crypt fission
- altered stem cell dynamics
- abnormal crypt fission
- enhanced unstable stem cell population
- crypts will be incrased in number, longer in length, increased branching
- cells more crowded, especially in the lower parts of the crypt

what is going on in this picture?

- top left corner has early adenomatous (dysplastic) crypt = begining of tubular adenoma
- rest has aberrant crypt fission, hyperplastic crypts
- due to both germline and acquired APC mutations
what will catenin expression look like in early adenoma? how will this change in late adenoma
- membranous, cytoplasmic and nuclear localization
- illustrates increase in free beta-catenin in the cytoplasm and transcriptional activation by beta catenin in the nucleus
- in late, will only be cytoplasmic and nuclear
- as cells lose junctional integrity, concentration of membranous catenin (junction associated) markedly diminshes
- d= early, f= late

how can NSAIDs be used theraputically for patients with colon cancer?
- have been found to prevent initiation of colon cancer/slow progression in pateints wtih FAP (and also with normal people)
- ligand for PPAR delta is blocked by NSAIDS => inhibits cell prolifferation effect of free beta catenin, permits cell death through apoptosis
- get reduction in polyps
how are prostaglandins related to colon cancer?
- elevated levels promote colon cancer
- if you give animals a mutation that increases PG levels, you get worse polyp formation
- 15-PGDH gene is often lost in FAP pateints
- 15 PGDH catalyzes NAD+-linked oxidation of 15 (S)-hydroxyl group of prostaglandins and lipoxins and is the key enzyme responsible for the biological inactivation of these eicosanoids.
what is celecoxib? how is it used?
- cox-inhibitor - reduces adenoma burden in retained rectum of patients after colectomy
- may reduce diminutive duodenal adenomas
- effects prostaglandin levels - inhibits cox-2, which normally increases PG levels


