F - Respiratory physiology Flashcards
Describe the structure and function of the upper airway (excluding the larynx)
The upper airway is comprised of:
- Mouth
- Soft palate + uvula form a valve to cut off the nasopharynx while eating
- Nasal cavity
- Turbinates - cirulates + warms air
- Secretion of mucous which filters air
- Sinuses
- Pharynx
- naso, oro + laryngopharynx
Describe the anatomy of the trachea (including its relations)
- 10cm long
- It is a conducting airway - ie. Does not take part in gas exchange
- Split into cervical and mediastinal portions.
- Mediastinal portion travels from anterior to posterior mediastinum
-
Borders + Relations:
- Upper border = larynx, begins at C6 & branches at the sternal angle (T4-5)
- Anteriorally, made up of 16-20 C-shaped cartilage separated by fibroelastic tissues.
- Posteriorally, made up of trachealis muscle
- Its relations on the right are lung & pleura
- Relations on left are descending aorta, lungs, pleura
- Relation inferior is the pulmonary trunk/right pulmonary artery
- Posterior relation is oesophagus
-
Histology:
- Made up of pseudostratified columnar ciliated epithelium, goblet cells (mucin secreting) + basal cells
- Blood supply: inferior thyroid artery & bronchial arteries + drains to inferior thyroid venous plexus
- Innervation: pulmonary plexus
Describe the anatomy + function of the larynx
(excluding blood/venous/lymph/nerves)
Functions = airway protection, speech + breathing, cough reflex
Relations
* Superior - hyoid bone
* Anteriorarly - skin and covered by thyroid cartilage
* Inferiorly - continuous with trachea at C6
* Posteriorly - projects into laryngopharynx
Structural anatomy
* Lined by pseudostratified columnar epithelium
* Divides respiratory tract into upper and lower
Cartilages - 3 paired, 3 unpaired
* Unpaired: epiglottis, thyroid, cricoid
* Paired: arytenoids, corniculate, cuneiform
Muscles - various muscles attaching to the various structures. Important movements:
* Phonation: cricothyroid (brings cords together by moving thyroid down), interarytenoid (transverse + oblique), vocalis (subset of muscles from thyroarytenoid) - mediates tension in vocal ligament to modulate pitch
* Inspiration: + cricoarytenoid (posterior + lateral) - rotate arytenoids outwards
* Expiration: thyroarytenoid adduct cords to increase resistance and provide intrinsic PEEP (3-4 cmH2O), which maintains patency of small airways & maintains FRC
* Effort closure - aryepiglottic muscles contract strongly to act as a sphincter, allowing airway to withstand up to 120cmH2O pressure
Ligaments
* Intrinsic - Cricothyroid ligament and quadrangular membrane
* Extrinsic: thyrohyoid membrane, median and lateral thyrohyoid, hyo-epiglottic, cricotracheal ligaments
Outline the anatomy of the bronchi + the bronchial tree to the level of the segmental bronchi. Briefly describe the anatomy of bronchioles
Bronchi
- The left and main bronchi split into lobar, then segmental, then small bronchi
- RMB shorter + wider than left
- The bronchi are cartilaginous + bronchioles are not
- Blood supply: bronchial arteries and pulmonary circulation
- Venous drainage: azygos + accessory hemiazygos vein
- Innervation: vagus + T2-6 sympathetic fibres
- Bronchial wall
- Made up of pseudostratified columnar epithelium, composed of goblet + basal cells (stem cells responsible for goblet + epithelial cell production)
- Basement membrane
- Submucous layer
Bronchioles
- Terminal bronchioles - cuboidal + ciliated epithelium
- Respiratory bronchioles - cuboidal and squamous epithelium
- Less goblet cells

Describe the structure of alveoli and relate it to its function
Macroscopic characteristics:
- Large no of airspaces connected by septae
- large SA to facilitate diffusion
- Interconnected network of walls allows mechanical stress to be shared across large area (alveolar interdependence)
- Pores of Kohn - allows collateral ventilation
- Blood-gas barrier (capillary endothelium-basement membrane- type I cell)
- short diffusion distance (0.2-0.5um) - high permeability to gas, low to water
- polyhedral shape
Histological features:
- Elastic basement membrane
- Increases elastic recoil of distended lung & increases resistance to atelectasis
- Capillary endothelium
- Alveolar epithelial cell Type I
- Make up most of the surface area & are the cells through which gas diffuses
- Alveolar epithelial cell Type II
- Responsible for surfactant production
- Granular pneumocytes
- Lamellar bodies (pools of phopholipids) are excreted + form tubular myelin, which then forms the phospholipid lining of the surfactant layer
- Replenish Type I cells (which cannot replicate)
- Pulmonary alveolar macrophages (PAMs)
- Phagocytose small partciles
- Can release lysosomal products into EC space in response to eg. Cigarette smoke/other irritants
What are the differences encountered in the upper airway for neonates, children + adults?
Anatomical airway differences are more prominent in children <12months old, and these differences become less pronounced at around age = 8
-
Head and neck - neonates have:
- Large occiput + proportionally short neck. Neck is flexed in supine position + favours airway obstruction in this position. Optimal intubation is in neutral vs ramped position
-
Oral + nasal cavity - neonates have:
- Smaller mandible - less anterior excursion + smaller mouth opening
- Large tongue - compared to size of oral cavity - interferes with intubation
- “Obligate nose breathers” - nasal obstruction will impair respiration
- Larger tonsils + adenoids - can cause airway obstruction. NPA may cause bleeding + aspiration
- Nil dentition
-
Larynx - neonates have:
- Large, floppy epiglottis - projects further into the airway + covers more of the glottis
- Superior laryngeal position - lies at C4 rather than C6 in adults
- Narrowest part of airway is at the cricoid, not the transverse diameter of the vocal cords as in adults
- Subglottic narrowing - can have FBs lodged below cords - resolves age 10-12
-
Trachea - neonates have:
- Short trachea - ~4cm. L & R bronchi arise at similar angles so easy for endobronchial intubation on either side. Accidental extubation also easier
- Soft trachea + cricoid - cricoid pressure may collapse airway
- Narrow - smaller target for needle/surgical cricothyroidotomy. Also risk of tracheal stenosis following prolonged intubation
Describe the anatomy + function of the diaphragm
Structural anatomy
- Complex dome shaped membranous structure with two discrete muscular portions (costal + crural diaphragm) + circumferential attachment (which allows diaphragm to increase intrathoracic volume)
- Skeletal muscle - predominately slow twitch fibres (to facilitate sustained contraction)
- Connects to lower six costal cartilages and posterior aspect of xiphoid process
- Three main tendons
- Central noncontractile tendon (level of xiphisternum)
- R crus
- L crus
- 3 arcrurate ligaments connect the diaphragm to the posterior abdominal wall
- Median
- Medial (over psoas)
- Lateral (over quadratus lumborum)
- Three perforations:
- T8 vena cava (8 letters)
- T10 oesophagus (10 letters)
- T12 aorta, thoracic duct, azygos vein
Innervation/blood supply/venous drainage:
-
Innervation:
- Motor: L + R phrenic nerves (C3, 4 and 5 keeps the diaphragm alive). Motor innervation solely from C3,4,5 – vulnerable to high spinal cord damage
- Proprioceptive: to periphery from lower intercostal nerves
- Blood supply: phrenic arteries from abdominal aorta
- Venous drainage: to IVC via tributaries of brachiocephalic + azygous
Function:
- Contributes to majority of inspiratory work of breathing
- Moves 1cm during tidal breathing
- Can move up to 10cm in forced breathing
- Can dramatically increase intrabdominal pressure (cough, sneezing, vomiting)
- Maintains lower oesophageal spincter tone
- Contraction: downward movement, flattening, tilt anterioposteriorly, increase circumference
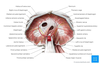
Describe the structure of the chest wall and its function in respiration
Chest wall is composed of:
- Ribs: antero-inferior slope, connected by intercostal muscles
- Intercostal muscles:
- Skeletal muscles
- External intercostals slope antero-inferiorly
- Internal + innermost intercostals slope infero-posteriorly
- Motor innervation from intercostal nerves at same level
- Function: bucket handle movement + elevation of ribs
- Incr diameter of thoracic cavity
- Minor muscles: levator costae (upper edge of rib to veterbral transverse process); transversus thoracis/triangularis sterni (? Function), scalene muscles (elevate rib case)
List the muscles involved in respiration
- Pharyngeal
- Genioglossus, palatal muscles, hyoid muscles
- Inspiration: Dilate the upper airway as reflex response to negative pressure
- Expiration: Relax passively
- Laryngeal
- Inspiration: Vocal cords abduct (decrease resistance to airflow)
- Expiration: Vocal cords adduct (increases airway resistance and prevents lower airway collapse
- Chest wall muscles
- Diaphragm (see diaphragm)
- Intercostals (see structure of chest wall)
- Scalenes, transversus thoracis
- Inspiration:‘bucket handle’ elevation of the ribs (mainly by external intercostals); ‘pump handle’ elevation of sternum
- Expiration: mainly internal intercostals
- Abdominal muscles
- Rectus abdominus, transversus abdominus, external + internal obliques, pelvic floor muscles
- Inspiration: apply counterpressure to flattening diaphragm to facilitate lateral + anteroposterior expansion of ches
- Expiration: Maintain intra-abdominal pressure + push diaphragm back up into chest. Active role whenever respiratory effort is increased
- Accessory muscles
- SCN, trapezius, pectoralis, extensors of the vertebral column, serratus anterior, latissimus dorsi
- Recruited to assist respiratory effort when energy requirements of ventilation are increased
Outline the anatomy of the pulmonary and bronchial circulations
Pulmonary
- Arises from pulmonary trunk
- Low pressure, highly elastric
- Blood supply: arises from bronchial circulation via vaso vasorum
- Nerve supply: SNS fibres>PSNS
- Structure:
- Elastic arteries - large, contain elastin. Less susceptible to changes in ITP
- Transitional arteries - less elastin & increasing amounts of muscle fibres running circumferentially
- Muscular arteries - enough smooth muscle to allow vasoreactivity
- Non-muscular arteries - small endotherlial vessels, which can be affected by transmitted alveolar pressures
- Capillaries - form a vascular sheet. This is the level at which gas exhange occurs
- Pulmonary veins return oxygenated blood to the LA. Thinner walled, contain more collagen & less elastic. Indistinguishable from LA endothelium & even contains myocytes (can be source of AF)
Bronchial circulation
- Arises from systemic circulation & forms the circulation for pulmonary malignancies
- R bronchial artery arises from an IC artery & on left there are usually 2 ateries with separate origins from the aorta
- Supplies blood to bronchi
Describe the relationship between PaCO2 & ventilation; & PaO2 & ventilation

Describe the features of central chemoreceptors
Medullary chemoreceptors
- Anatomically separate to medullary respiratory centres
- On ventral surface of medulla (~200-400microns deep to surface)
- Surrounded by brain ECF, with CSF next to the ventral surface, and blood vessels on the other side of chemoreceptors - ie. The pH changes depend on CSF, local blood flow, ECF
- The blood-brain barrier is relatively impermeable to ionic H+ & HCO3 -, but molecular CO2 diffuses across easily. This then contributes to release of H+ ions which results in decreased pH
- Normal CSF pH is 7.32. It has lower buffering capacity than blood due to lower protein count, therefore there is a greater difference in CSF pH compared to blood pH for any given change in PCO2. It also responds more quickly to renal compensation - it therefore has a more important effect on the level of ventilation and arterial pCO2.
- Eg. In CO2 retainers (COPD), chronic pCO2 change results in compensatory increase in HCO3- & CSF pH approaches neutral. This causes a lower respiratory rate than would be expected with the observed arterial pCO2
Describe the peripheral afferents involved in respiratory control
Lung receptors (all impulses travel via vagus)
- Pulmonary stretch receptors - discharge in response to distension of lung & activity is sustained with lung inflation - ie. They show little adaptation
- Stimulation of these receptors results in slowing of respiration due to increase in expiratory time.(Hering-Bruer reflex)
- opposite is true for expiration
- Irritant receptors - rapidly respond to airway irritants - eg. Cigarette smoke/noxious gases/cold air
- J receptors - respond to chemicals injected into the pulmonary circulation –> results in rapid, shallow breathing
- Bronchial C fibres - respond to chemical injected into the bronchial circulation –> results in rapid, shallow breathing
Other receptors:
- Nose + upper airway receptors
- Joint + muscle receptors
- Thought to provide feedback to ventilatory centres via proprioceptive info
- During exercise, descending control of muscle activity may stimulate the central respiratory control centres
- Pain + temperature
- Temperature increases the sensitivity of peripheral chemoreceptors to O2 - rise in temp will increase minute volume at any given PaCO2 + PaO2
- Baroreceptors
- May also have a role in ventilation - hypertension increases respiratory rate while hypotension decreases it
Describe the control of breathing

Describe transmural pressure and its role in the inspiratory and expiratory process
Intrapleural pressure:
- Space between the lung and the chest wall (or between visceral and parietal pleura
- Balance between outward recoil of chest wall + inward recoil of lungs
- Usually negative –> -5cmH2O at rest
- Varies with vertical distance in the lung
- Gravity pulls lung parenchyma inferiorly
- IPP therefore more negative at apex (typically -10cmH2O at FRC), less negative at base (typically -2.5-3cmH2O at FRC)
- During inspiration, pleural pressure changes evenly throughout the lung, however basal alveoli are better ventilated because their compliance is increased (due to lower resting volume)
Inspiration
- Negative IPP (-8cmH2O); Ppl > Pel (pl = IPP, el = elastic recoil of lungs)
Expiration
- Ppl falls to -5cmH2O

Define compliance
Compliance:
- Measure of the ‘distensibility’ of lung - change in unit volume per change in unit pressure (see equation 1)
- Compliance of the lung: equals transpulmonary pressure = alveolar pressure - Intrapleural pressure
- Compliance of the chest wall: = intrapleural pressure - ambient pressure (usually atmospheric)
- Total compliance is calculated from the alveolar-ambient pressure gradient
- Elastance = 1/compliance (the elastic recoil)
- Compliance of the respiratory system as a whole is a function of both lung and chest wall compliance: (see equation 2)
- In the normal range (-5 to -10cmH2O), both lung and chest wall compliance is independently stated as 200ml/cmH2O, therefore compliance of the respiratory system as a whole is 100mL/cmH2O
Static compliance
- Compliance in the absence of flow - ie. Compliance of the system at any given volume when there is no flow
- It is a function of elastic recoil of the lung and surface tension of alveoli
- In ventilated patients, this can be measured by tidal volume/(Pplat - PEEP)
Dynamic compliance
- Measured during respiration, using continuous pressure and volume measurements
- Includes pressure required to generate flow by overcoming resistance forces - therefore always less than static compliance
- It is a function of respiratory rate
Specific compliance
- Compliance per unit volume of lung (see equation 3)
- This is usually ~ 0.05/cmH2O - this is used to compare difference sized lungs. It is the same between adults and neonates
- Lung compliance exhibits hysteresis (compliance is different in inspiration and expiration)
- In static compliance curves - hysteresis is due to viscous resistance of surfactant and the lung
- In dynamic compliance curves - hysteresis is due to airways resistance (which is a function of flow rate), which is maximum at beginning of inspiration and end-expiration

Describe the different ways of measuring compliance
Static compliance:
- Supersyringe method - large syringe used to titrate known volumes of air into lung (usually increments of 100mLs). The pressure at each of these volumes is measured (?oesophageal balloon)
- Limitations:
- Gas is compressible - (not taken into consideration) at higher pressures, some volume will be lost (compliance will look better)
- Ventilator has to be disconnected to attach syringe - may lose some PEEP (not measuring true compliance)
- Method takes a long time - ~2-3 seconds are allowed for diffusion prior to measuring pressure therefore some volume of gas may be absorbed (compliance will look better)
- Temperature changes - heated/humidified gas will expand (compliance will look worse)
- Limitations:
- Multiple occlusions method - during normal ventilator function, breath occlusions are repeated at different volumes with normal breaths in between (eg. Breath hold at 200mLs, measure pressure, then normal breaths + breath hold at 400mL, etc)
- Doing an inspiratory pause in a pt with mandatory ventilation - compliance is measured by Vt/(Pplat - PEEP) - this is measuring compliance at the highest volume of that breath
- Continuous flow method - Low rates of inspiratory flow used on ventilator, in attempt to reduce respiratory resistance (~1.7L/min) over 10-15seconds, followed by low expiratory flow rate
- Tends to underestimate inspiratory compliance (due to airway resistance) & overestimate expiratory compliance
- Limitations of all methods of static compliance - all pts need to be sedated + paralysed, possible escape of gas into pulmonary circulation, ignores changes in gas pressure + temp
- Pressure in ventilators is measured using integrate silicon waver transducers. An aneroid manometer may be used to measure pressure during supersyringe inflation/deflation
Dynamic compliance:
- Occurs during normal ventilator function - makes no attempt to correct for pressure produced by airway resistance
- Built into modern ventilators
What are the factors affecting compliance?

What are the properties of surfactant?
- Surfactant is 85% phospholipid lecithin (dipalmitoyl phosphatidylcholine DPPC major constituent -produced by Type II alveolar epithelial cells); 10% protein; 5% neutral lipid
- DPPC = amphipathic
- hydrophobic at one end and hydrophilic at the other end
- align themselves on the surface
- their intermolecular repulsive forces oppose the normal attracting forces between liquid molecules that are responsible for surface tension
- Surfactant release stimulated by: catecholamines, cholinergic, vasopressin, adenosine
- Surfactant is inhibited by high conc surfactant proteins, lectins, inflammatory mediators
- Produced by Type II alveolar cells + secreted via lamellar bodies
- T1/2 = 5-10 hours; removed by: resorption to Type II cells, transported up airways and eliminated as aerosol; degraded in alveolar macrophages, extracellular enzymatic degradation, clearance via lymph / blood
What is surface tension?
Surface Tension is the force that arises due to attractive forces (hydrogen bonds in water) between adjacent molecules of liquid being much greater than those between gas and liquid; where liquid surface area becomes as small as possible
- Liquid surface area contracts as much as they can (i.e into a bubble) and generates a pressure as per LaPlace Law
- P = 2(gamma) / r P = pressure inside elastic sphere
- Thus, at any given (gamma)(surface tension), reduced r -> higher transmural pressures (want to collapse more)
- Smaller alveoli promote their own demise by emptying into larger neighbouring alveoli
- High surface tension causes three problems
- Compliance falls when alveolus is empty
- as r falls pressure required to open it at given is increase -> incr work of breathing
- Small alveoli will preferentially empty into bigger alveoli
- smaller alveoli require greater transmural pressure to remain inflated -> causes smaller alveoli to empty into larger ones
- Fluid transudation
- surface tension draws fluid from interstitial spaces and contributes to pulmonary oedema
How does surfactant influence respiratory mechanics?
Functions of Surfactant
- Reduce surface tension – increase lung compliance + reduce work
- Alveolar surface tension decr to virtually zero – particularly when alveoli deflate and phospholipid particles are brought closer together
- increase lung compliance from decr surface tension
- Alveolar stability + interdependence
- when alveoli are fully inflated, surfactant phospholipid molecules are farther apart, which decr compliance on lung deflation -> hysteresis (compliance is different in inspiration + expiration)
- Reduce alveolar transudate
- decreased surface tension -> decreased capillary-alveolar hydrostatic pressure gradient, decr ultrafiltration of fluid

What are the factors that influence FRC?
See table

Describe the factors that affect airways resistance
Can be derived from components of Reynold’s number and Hagen-poiseuille equation
- Factors that affect gas properties - eg. Viscosity and density
- Factors that affect airway diameter
- Intraluminal e.g. sputum plugging, airway oedema, water in circuit, low lung volumes (eg in anaesthesia - airways tend to collapse)
- Luminal (Tone): SNS b2 receptors in lung cause bronchodilation, PSNS causes bronchoconstriction via musc R
- Extraluminal: Dynamic airway compression (Starling resistor) e.g. on forced expiration (occurs in COPD as well due to ¯ elastin), artificial airway
- Factors affecting length
- Tracheostomy - decreases airway length; ETT - elongation of large airways at high volume
- Factors affecting flow rate:
- Respiratory rate ( RR = turbulence as seen with Reynold’s number)
- Inspiratory/expiratory work/effort - as in forced expiration
List the ways of measuring airway resistance + how flow changes can be detected
Measurement of respiratory resistance
- Direct measurement of air flow, airway pressure and alveolar pressure (eg. Oesophageal balloon)
- Body plethysmography
- Forced oscillation technique
- Airway resistance interrupter technique (Rint)
- Inspiratory hold (in mechanically ventilated patient)
- Rhinomanometry
Methods of determining flow + detecting increased resistance to flow
- Spirometry
- Mechanical ventilator data
- End-tidal gas monitoring
Describe and explain the oxygen cascade
The Oxygen Cascade describes the drop in the partial pressure of oxygen from the atmosphere to the mitochondria
- Atm 159mmHg at sea level + 37OC (Or, 21% of total atmospheric pressure of 760mmHg)
-
Airway 149mmHg
- Drops due to water vapour pressure
-
Alveolar 99mmHg
- Drops due to oxygen uptake by pulmonary capillaries + CO2 influx into alveoli
- Cannot be measured - calculated by alveolar gas equation
- End-capillary ideally the same as Alveolar
-
Arterial (PaO2) is 92mmHg
- Drops due to venous admixture
- Degree of drop = A-a gradient (7 = normal in adults - up to 14 can be normal in older adults)
- Tissue - varies between tissues (10-30mmHg) - brain is 33mmHg. Drops due to diffusion distance
- Mitochondrial - drops due to diffusion distance. Between 1-10mmHg
Explain time constants, what is meant by the term ‘pendelluft’ and its effect on ventilation
- ‘Time constant’ is a term derived from mathematics and applied to respiratory physiology to describe the filling/emptying behaviour of alveoli with varying properties:
- One time constant (tau) refers to the time it takes for an alveolus to fill/empty by 63% of its total amount
- It takes 3 time constants for an alveolus to fill/empty by 95%
- For normal lungs, an expiratory time constant is usually given as approx. 100-200ms, so that over 0.6s, 95% of volume should be emptied
- tau = compliance X resistance (given flow is constant)
-
‘pendelluft’ refers to the equilibration process between ‘fast’ and ‘slow’ alveoli via interconnectedness (pores of Kohn)
- Can be observed via inspiratory hold on ventilator - initial drop in pressure (airways resistance), followed by longer/slower downtrend in plateau pressure (as alveoli equilibrate)
- Examples via disease processes:
- Pulmonary fibrosis: is same or decreased “fast”, compliance is decreased, resistance increased
- Emphysema: is increased “slow”, compliance increased, resistance increased
- Effect on ventilation:
- At the beginning of expiration, the abnormal region may still be inhaling while the rest of the lung has begun to exhale with the result that gas moves into it from adjoining units (pendelluft - swinging air)
- As breathing frequency increases, the proportion of tidal volume that goes to the partially obstructed region becomes smaller - less of lung participates in TV changes & lung appears to become less compliant

Explain the significance of the vertical gradient of pleural pressure and the effect of positioning
Compared to alveoli at the base, alveoli at the apex:
- Are larger at end-expiration
- Have lower ventilation + perfusion but higher V/Q ratio
- This results in differences in gas composition. Compared to basal alveoli, apical alveoli have pO2 132mmHg vs 89mmHg(base); pCO2 29mmHg vs 42mmHg (base)
- The differences in oxygen uptake and CO2 output result in higher respiratory exchange ratio at apex (RER = 2) vs base (RER = 0.67)
- There is a difference in hydrostatic pressure of the between the top and bottom of the erect lung of 30cms H2O (or 23mmHg)
- Perfusion differences due to gravity are described by wests zones
- Ventilation differences are an indirect consequence of the effect of gravity
- Intrapleural pressure gradient - the weight of the lung causes a gradient of intrapleural pressure between the top and bottom of the lung (-10cmsH2O & -2.5cmsH2O). Greater negative pressure of apical alveoli causes greater distention (and thus greater size)
- Ventilation gradient - gradient in alveolar sizes at FRC means that alveoli will increase in size by different amounts with inspiration because they are on different parts of the lung’s compliance curve. Smaller basal alveoli increase in size more than apical
- Note: if a person is tidal breathing from just above RV (low lung volumes), IPP at apex is decreased to -4cmsH2O, base to +3.5cmsH2O (constant gradient of 7.5cmsH2O as lung weight unchanged). However, ventilation at apex is better than base (positive pressure at base results in airway closure - this represents a move along the curve as outlined (image below)
*Note: the V/Q gradient exists because the vertical gradient in perfusion is larger (steeper) than the vertical gradient in ventilation*

Describe the pressure and volume relationships in the respiratory system
Pressure + volume loops in the respiratory system display hysteresis (see below)
This is due to the effect of surface tension on lung mechanics

Describe how the pressure-volume loop changes as compliance decreases

Describe the flow-volume relationship of the lung

Describe the work of breathing and its components
Work of breathing = Pressure x Volume (measured in Joules)
- This gives the work for a single respiratory cycle. Tidal breathing is efficient and uses <2% of BMR
- In a normal person at rest, WOB is 0.35J/L
- Energy expenditure over time is called the “power of breathing” and is roughly equal to 2.4J/min
- The oxygen requirement of breathing at rest is 2-5% of VO2 or 3ml/min
Components of the work of breathing:
- Elastic work - about 65% of total work and stored as elastic potential energy. The energy required to overcome elastic forces are:
- Elastic recoil of the lung
- Elastic recoil of the chest
- Resistive work - about 35% of total work & is lost as heat. This is due to energy required to overcome frictional forces:
- Between tissues (increased with interstitial lung disease)
- Between gas molecules - increased with high flow rates, turbulent flow (rate, density), decreased airway radius (eg. Low lung volume/inadequate PEEP, bronchoconstriction, apparatus)
The below refers to attached graph:
- The area between the compliance line and the inspiratory line is additional resistive inspiratory work done
- The area between the compliance line and expiratory line is additional resistive expiratory work done
- This is work typically done by elastic recoil of lungs
- If this area falls within the area of elastic work of breathing, it is a purely passive process (using stored elastic potential energy of inspiration).
- If part of the area falls outside the area of elastic work, it demonstrates additional active work of expiration, which may occur in obstructive lung disease or when minute ventilation is high

State the normal lung volumes and capacities

Describe the nitrogen wash out method of determining total lung capacity, including the inaccuracies with this method of measurement
- Subject with air in lungs is commenced on 100% FiO2.
- Then begins to exhale nitrogen with every breath and nitrogen content in their exhaled gas mixture decreases with every breath
- This nitrogen content is then measured as a percentage of exhaled volumes
- Nitrogen washout continues until all nitrogen is replaced by oxygen - classically takes about 7 minutes (70-80 tidal volumes)
- From the total nitrogen content and first measured breath’s nitrogen concentration, it is possible to determine total lung volume
- Inaccuracies: N2 concentration is extrapolated from the measurement of O2 and CO2 in expired gas; there may be leaks in the system. Some N2 exhaled does not come from lung volume but from body fluids and tissues; if there is gas trapping, tapped gas will never escape to be measured and the nitrogen washout method will underestimate lung volume
Describe the tracer gas dilution method of measuring TLC
- Give a subject a bolus of tracer gas, with a known volume which contains a known concentration (eg. 100%) of tracer gas. The subject inhales this volume and it is distributed widely and evenly in their lung volume
- The subject then exhales their intrathoracic gas. The concentration of this gas is measured
- From this concentration, the volume of distribution of that gas can be calculated (ie. Intrathoracic volume):
C1 x V1 = C2 x (V1 + V2)
- C1 = Initial tracer concentration in the bolus
- V1 = volume of the bolus
- C2 = tracer concentration in the exhaled gas
- V2 = volume of the lung
Describe body plethysmography
- Consists of subject sitting in a closed box (with known volume), connected to a mouthpiece
- When the subject takes a breath in, the expansion in the chest wall displaces/compresses the volume of gas in the box, which causes a corresponding increase in box pressure (which can be measured), according to Boyle’s Law (V.P = constant). The opposite happens with exhalation
- When the subject inhales against an obstructed mouthpiece (airway), the negative pressure at the mouth can be measured and used to infer the lung volume.
Outline the factors that alter closing capacity
- Decreased surfactant - anything leading to increasing tendency for alveoli to collapse will result in increase of closing capacity
- Expiratory effort - increase in expiratory effort will increase intra-alveolar pressure (secondary to chest wall pressure). This may cause intra-alveolar pressure to exceed negative intrathoracic pressure
- Small airways disease (eg. Asthma, COPD) - increases CC due to increased muscular tone/mucous production leading to airways staying open at higher volumes
- Age
- As age increases, there is less elastic stretch to keep airspaces open
- CC = FRC in neonates
- CC = FRC in supine 44yo
- CC = FRC in erect 60yo
Outline the clinical significance and measurement of closing capacity
Clinical significance:
- Increased CC decreases the effect of pre-anaesthetic preoxygenation (difficult to denitrogenate collapse airways)
- Increased CC increases dependent atelectasis
- Which can in turn cause lung injury via cyclic atelectasis (eg. In the setting where CC overlaps with tidal volume - airspaces may start closing at the end of normal expiration, and the repeated open and closing of these airspaces may cause injury similar to VILI)
- Responsible for age related decrease in PaO2 over time
How is it measured?
- Fowler’s method can be carried out with either tracer gas (xenon) or resident gas (nitrogen)
- Method:
- Patient expires to RV (residual volume). At this volume, dependent airways have collapsed and upper lobe (non-dependent) airways remain open with nitrogen-containing air
- Patient inspires 100% FiO2 to TLC
- Patient expires and nitrogen concentration of expired gas is measured:
- Phase I - dead space air, containing no N2 comes out
- Phase II - increasing N2 containing gas comes out as alveoli are emptied
- Phase III - N2 concentration plateaus as N2 containing air from upper lobe mixes with pure 100% O2 from dependent airways, creating a constant concentration of N2
- Phase IV - as dependent airways collapse, N2 concentration of expired gas rises again (no more mixing with 100% O2 containing gas).
- The point at which N2 starts to rise again is the closing volume
*closing capacity = closing volume + residual volume

Describe the balance between hydrostatic and oncotic pressures in the pulmonary circulation
(Starling equation below). In the pulmonary circulation, these are:
- LpS - surface area ~140m2 (compared to 4000-7000m2 in systemic circulation)
- Pc = 4-12mmHg
- Pi = interstitial hydrostatic pressure - essentially equal to alveolar pressure = atm pressure. Increases during PPV
- Surfactant decreases hydrostatic pressure by decreasing surface tension
- Pi(c) = 25mmHg throughout circulation; affectd by blood protein count
- Pi(i) = ~3mmHg at alveoli
- Sigma = 0.5-0.7 in lung

Define pulmonary vascular resistance and outline the factors affecting it
- Defined as the resistance to blood flow for a given pressure gradient across a vessel
Factors that affect PVR:
- Recruitment + distension
- At normal pressures and flows, some pulmonary capillaries are partially narrowed or collapsed
- As blood flow increases, capillaries that were collapsed are recruited, and capillaries that are narrowed are distended
- Lung volume
- PVR and lung volume have a ‘U’ shaped relationship, with lowest PVR at FRC
- At larger lung volumes, smaller capillaries are compressed. At smaller lung volumes, larger vessels/capillaries are collapse
- Hypoxia
- Hypoxia causes vasoconstriction of the pulmonary capillaries - this increases PVR
- Hypercapnoea causes vasoconstriction to a degree as well
- Acidaemia causes pulmonary vessels
- Gravity
- Blood has to flow against gravity to reach the upper lobes & resistance to flow is higher. Resistance to flow is lower in the middle + lower lobes (phenomena known as West’s zones)
- Autonomic nervous system + drugs affecting it
- α1 receptors - vasoconstriction
- β2 receptors - vasodilation
- M3 muscarinic receptors - vasodilation
- Local mediators
- Vasoconstriction - histamine, endothelin, serotonin
- Vasodilation - nitric oxide, prostacyclin, isoprenaline
**Note: should reproduce below graph when talking about PVR**

Compare the differences between the pulmonary and systemic circulations
Table

Describe the causes for differences in regional ventilation and perfusion & display on a graph how ventilation and perfusion are distributed throughout the lung
Ventilation:
- Global - minute volume is average of 4L/min (eg TV 400mL & RR 10)
- Regional - there may be multiple different reasons for uneven ventilation:
- Gravity-related vertical gradient of pleural pressure
- Posture (changes the direction of this gradient)
- Bases have more room to expand than apices
- Compliance differences between lung regions
- Pattern of breathing - it has been shown that there is a difference in pleural pressure distribution & radioactive xenon washout between breathing which engaged intercostal muscles as compared to ‘routine’ diaphragmatic breathing
Perfusion:
- Global - roughly equal to cardiac output
- Regional - regional blood flow is affected by multiple factors:
- Variation in alveolar oxygenation (hypoxic pulmonary vasoconstriction)
- Gravity related hydrostatic pressure
- Basic architecture of pulmonary vessels - this appears to be pretty heterogenous

Describe West’s zones of the lung and explain the mechanisms responsible for them
West’s Zones of the lung refer to the differences in perfusion between the apex and base of the upright lung due differences in hydrostatic pressure
Zone 1
- Refers to zone of the lung where alveolar pressure is greater than pulmonary arterial pressure
- Flow stops due to pulmonary arterioles/capillaries being squashed by alveoli, resulting in physiological dead space
- This does not occur under normal physiological conditions, but can happen in conditions that either increase pulmonary alveolar pressure or decrease pulmonary arterial pressure, or both, such as:
- Positive pressure ventilation
- Haemorrhage or other causes for decreased intravascular volume
Zone 2
- Refers to area of lung in which pulmonary arterial pressure is greater than alveolar pressure, but alveolar pressure is greater than pulmonary venous pressure
- Alveoli in this zone are intermittently be perfused during expiration
Zone 3
- Area of lung in which both pulmonary arterial and venous pressure is less than alveolar pressure
- Flow is determined by pressure gradient between pulmonary arterial system and pulmonary venous system
Zone 4
- Base of lung where flow is determined by the interaction between pulmonary arterial pressure and interstitial pressure. (Pulmonary artery is squashed by interstitial forces)
Explain ventilation-perfusion matching and mimatching
Ideally, perfusion would be matched to ventilation (V/Q = 1) & gas exchange would be ideal under these conditions. Due to differences in blood and gas supply to different lung regions (see above), there is a range of V/Q ratios from 0-infinity
- When plotting blood/gas flow against V/Q ratio, you get a bell curve
- Typical minute ventilation = 4L/min & typical CO is about 5L/min, so global V/Q ratio would be about 0.8, if the whole lung was averaged
- Below is from a 22yo male
- V/Q ratios generally range between 0.3-3.

Define dead space and its components. Explain how these may be measured and describe the physiological impact of increased dead space
Definition: Dead space is the fraction of tidal volume which does not participate in gas exchange. It can be classified in multiple ways:
- Anatomical dead space:
- Volume of the conducting airways
- This is equal to about 150mLs in an adult (~2.2mLs/kg), higher in infants (~3mL/kg)
- Nunn - decreases in tidal volume will decrease the amount of dead space proportionately
- Physiological dead space:
- The part of tidal volume which does not participate in gas exchange
- This definition is designed to include alveoli that may be well ventilated but not so well perfused (for whatever reason)
- In healthy adults, physiological and anatomical dead space will be pretty similar & the gap (alveolar dead space) widens in pathology
- It includes anatomical dead space + alveolar dead space
- Apparatus dead space - related to volume within ventilatory devises eg. ETT/trache
How to measure dead space:
- Anatomical dead space - can be measured by Fowler’s method –> with initial breath of 100% FiO2 to flush out nitrogen
- Anatomical dead space will contain 100% O2 and 0% nitrogen
- Alternatively, can use CO2 instead of N2 for Fowlers - all CO2 containing air will have to have come from areas of the lung that participated in gas exchange. This will measure physiological dead space
- Physiological dead space calculation - can use the Bohr (/Enghoff) equation to calculate this (see pic)
Physiological impact of increased dead space:
- As dead space is a fraction of tidal volume not undergoing gas exchange, increasing dead space would be effectively the same as reducing tidal volume
- Increasing alveolar dead space (eg. In PE) will increase your minute volume proportionally to the change in the ratio of dead space to alveolar ventilation

Explain the concept of shunt, its physiological effects and its measurement
Shunt refers to alveoli that are perfused but un-(or under-)ventilated.
- It is sometimes used interchangeably with venous admixture
- “True” shunt describes lung units were V/Q = 0; whereas lung units with a V/Q <1.0 will contribute to total venous admixture
Measurement of shunt:
- Shunt/venous admixture can be measured by the Berggren equation
- “true” shunt can be separated from venous admixture by breathing in 100% FiO2. Poorly ventilated alveoli will receive higher O2 content so that the end capillary O2 content of these units will be very similar to systemic arterial blood. This eliminates V/Q scatter
- For lung units with ‘true’ shunt, no amount of extra inspired O2 will improve oxygenation
- ABG machines can calculate shunt fraction by modifying Berggren equation & making calculated assumptions about the venous PaCO2
Physiological effects:
- Hypoxaemia
- Venous admixture gives rise to systemic hypoxaemia proportional to the shunt fraction
- A shunt fraction of 25% can drop O2 sats to 90s (PaO2 65mmHg)
- CO2 clearance
- Shunt doesn’t make much of a difference to CO2 clearance. In large shunt states, you will likely get increased minute ventilation due to central control which will increase CO2 clearance
Explain venous admixture, its relationship to shunt and ventilation-perfusion (V/Q) mismatch. List the causes of venous admixture
Definition: a volume of blood that needs to be added to ideal (ie lung unit where V/Q = 1) pulmonary end capillary blood to explain the observed difference between pulmonary end capillary oxygen content and arterial oxygen content. Ie. It is a calculated volume which appears to have bypassed the pulmonary gas exchange surface
- Often used interchangeably with ‘shunt’, however ‘true shunt’ in the pulmonary system refers to lungs with V/Q = 0
- Can’t measure shunt directly (can’t separate V/Q= 0 vs scatter) so calculate venous admixture
Calculation: measured by the shunt equation (Berggren equation - shown below). The shunt fraction is the ratio of venous admixture to total cardiac output
- Normal shunt fraction/venous admixture is 3%
Increased venous admixture occurs when perfusion (Q) exceeds ventilation (V), resulting in a V/Q mismatch, where V/Q< 1.0
Causes:
- Physiological/anatomical - sometimes referred to as ‘true anatomical shunt’
- Thebesian veins
- Bronchial veins
- Pathological
- Intrapulmonary - pulmonary AVM or fistula; anoxic lung tumour
- Intracardiac - ie congenital heart disease
- Other - porto-pulmonary shunt in liver disease (sats increase in supine vs standing)
- Either pathological or physiological
- True shunt - lung units with V/Q = 0. A certain amount of lung units will be like this under physiological conditions, with an increasing number with age (due to raised closing capacity), however this can also occur in disease (pneumonia/atelectasis/etc)
- V/Q scatter - lung units with V/Q<1.0. Similar to above

Explain the effect of ventilation-perfusion mismatch on oxygen transfer and carbon dioxide elimination
The V/Q ratios throughout the upright lung vary (see figure 1):
- V/Q= infinity –> dead space ventilation
- infinity > V/Q > 1 –> towards apicies
- V/Q = 1 –> ideal & occurs in the mid zones of the lung at ~ 3rd rib
- 0 towards bases
- V/Q = 0 –> true shunt
Effect on gas exchange (see figure 2):
- As lung units have increasing V/Q ratio, the closer the composition of pulmonary veins approaches that of alveoli
- As V/Q decreases below 1 & approaches zero, the closer the partial pressure of oxygen in pulmonary venous approaches that of mixed venous blood
- The effect of the V/Q ratio dropping from 1 to 0.1 causes a comparatively large drop in PaO2 and small rise in PaCO2
- The relationship between PaO2 and V/Q is steeper and more sigmoid than the relationship between PaCO2
- The greatest useful improvement in gas exchange occurs in the V/Q range of 0.1-1.0 (usually in bases of lungs) - small change in V/Q yields substantial improvement in oxygenation

Outline the methods used to measure ventilation-perfusion mismatch
To assess global mismatch:
- MIGET (Multiple inert gas elimination technique)
- Several gases with different solubility in blood are prepared. From most to least soluble:
- Acetone
- Ethanol
- Cyclopropane
- Enflurane
- Ether
- Sulphur hexafluoride
- These are dissolved in saline or dextrose and infused into the patient
- Each of these gases has a different partition coefficient (lamda- represents the ratio of concentrations of the gas in blood and alveolar gas at equilibrium
- For each of these gases, the ratio of arterial to mixed venous partial pressure can be calculated
- Ratio will decrease as V/Q increases (more gas left in circulation as perfusion decreases)
- For a gas with a given lamda, it is best suited to interrogate alveoli where the V/Q ratio approximates lamda
- For alveoli with higher V/Q ratio than lamda, most of the gas will be left in the blood
- For alveoli with lower V/Q ratio than lamda, most of the gas will be exhaled
- Multiple gases are use to assess a large range of V/Q ratios
- Several gases with different solubility in blood are prepared. From most to least soluble:
- Riley method (three-compartment model)
- V/Q scatter can be classified into 3 lung units:
- V/Q = 1.0
- V/Q = 0 (“true” shunt)
- V/Q = ∞
- As gas exchange occurs only in the ideally match unit, all changes in arterial and alveolar gas mixture as due to events taking place in this unit, so only measurements you need to make is alveolar O2 + CO2 & arterial O2 + CO2
- V/Q scatter can be classified into 3 lung units:
Imaging techniques (regional mismatch)
- MRI
- Gadolinium IV contrast used to image vessels
- Tracer gas (oxygen, 3He or 129Xe) can be used to image gas in the lungs
- SPECT (V/Q scan - can be used with or without CT)
- (Single proton emission computed tomography)
- Regional distribution of radionuclides is measured by a camera which detects gamma rays
- Perfusion measure using IV 99mTc-labeled macroaggregated albumin
- Ventilation measured using 133Xe
- PET
- PET has higher sensitivity than SPECT
- Same technique as SPECT but with use of positron-emitter isotopes (13N2) instead of gamma-ray emitters
Describe the movement of carbon dioxide from the cell to the atmosphere
Intracellular:
- CO2 tension between mitochondria and tissues are similar, due to the high solubility of CO2
- PCO2 ~ between 46-50mmHg
Tissues:
- Tissues can be classified by how fast CO2 equilibrates with the bloodstream:
- Fast compartment:
- Brain, kidneys, exercising muscle
- Slow compartment:
- Bone, fat, cartilage
- Intermediate compartment
- Resting muscle
- Fast compartment:
Bloodstream:
- Partial pressure of CO2 in capillaries probably variable and strongly dependent on the metabolic activity of the tissues surrounding that capillary
- Transported to lungs via bicarbonate, carbamates and dissolved in blood
- Mixed venous blood: ~46mmHg (or 6mmHg higher than arterial)
Alveolar CO2:
- Essentially the same as pulmonary capillary CO2 due to high diffusive capacity of CO2 and small membrane
- ~50mmHg
- Higher than mixed venous blood due to reverse Haldane effect (CO2 release from haemoglobin and bicarbonate stores)
Atmospheric CO2:
- 0.3mmHg
Explain perfusion-limited and diffusion-limited transfer of gases
Diffusion limited:
- Rate of gas uptake in capillary is determined by the rate of diffusion across the blood-gas barrier
- Rate of diffusion from alveolus to blood is very slow
- For all the length of the capillary, the gradient between the alveolus and the blood remains high
- An increase in the capillary blood flow rate will have minimal effect on gas uptake
- An increase in the partial pressure gradient between the alveolus and the capillary will increase the rate of diffusion
- Eg. Carbon monoxide
Perfusion limited:
- Rate of gas uptake in the capillary is determined by capillary blood flow
- The rate of gas diffusion into the capillary is very rapid
- Equilibration between the alveolus and capillary occurs shortly after blood enters the alveolar capillary
- For most of its length, the capillary blood is fully saturated with the gas
- Increasing the blood flow rate will increase the rate of total gas uptake, until the capillary transit time is faster than the gas diffusion time
- Increasing the partial pressure gradients between the alveolus and capillary does not significantly increase the rate of gas uptake into the blood if the blood flow remains the same
- Eg. O2, CO2 & nitrous oxide

List the physiological factors affecting the diffusion of oxygen across the alveolar membrane. Describe how this changes with exercise.
DLO2 = oxygen uptake/PO2 gradient
(measured in mL/min/mmHg)
[* Note - DL is closely related to Fick’s Law of diffusion. DL = AK/T (or AK/dx), so conceptually, Fick’s Law can be rearranged to give: DL = -J/dx]
Factors affecting diffusion of gases across the blood-gas barrier:
- Factors affecting gas properties (“D” (diffusion coefficient))
- Molecular size
- Density + viscosity
- Temperature
- Factors affecting the gas exchange surface area (“A”)
- Age
- Body size
- Lung volume
- Shunt, dead space, V/Q inequality
- Factors influencing membrane characteristics (“dx”)
- Disease states - eg. Pulmonary oedema, pulmonary fibrosis
- Factors affecting uptake by erythrocytes
- Affinity of Hb for O2
- Hb concentration
- Cardiac output (capillary transit time)
In exercise:
- Oxygen uptake increases
- Increased cardiac output –> increased pulmonary blood flow
- Better V/Q matching - perfusion of previously non-perfused areas
- Increased tidal volumes (Surface area increases)
- Partial pressure gradient “dP” increases
- Oxygen extraction ratio increases (Mixed venous PO2 will be lower)
- Increased minute ventilation will cause decreased PACO2 + therefore increased PAO2
- Increased delivery of Hb to absorptive surface acts as an oxygen sink and maintains a low capillary partial pressure

Define diffusing capacity and describe its measurement
Definition of diffusing capacity
- The propensity of a gas to diffuse as a result of a given pressure gradient
- Diffusing Capacity (DL) = net rate of gas transfer/partial pressure gradient
- For oxygen, this can be expressed as:
- DLO2 = oxygen uptake/PO2 gradient
[* Note - DL is closely related to Fick’s Law of diffusion. DL = AK/T (or AK/dx), so conceptually, Fick’s Law can be rearranged to give: DL = -J/dx]
- DL is measured in mL/min/mmHg
Measurement of diffusing capacity
- Practically, it is easier to measure DLCO than DLO2, as is CO rapidly bound to Hb & PCO gradient will be equal to the alveolar PCO partial pressure (which is the known exhaled partial pressure measurement)
- The CO uptake will just be inhaled minus exhaled
- Single breath method of measuring DLCO:
- Pt exhales maximally to RV
- Inhales 0.3% carbon monoxide & 10% helium (helium is for measurement of alveolar volume) - inhales to TLC
- Breath hold for 10s
- This is ensure equal distribution of CO to all lung units, irrespective of time constants
- Pt then exhales
- Gas sampled
- Total alveolar volume measured from expiratory helium concentration
- Rebreathing method of DLCO*:
- Essentially same as above but nil breath holding
- Pt breathes rapidly (RR = 30), while breathing from a reservoir with known quantity and volume of gas with 0.3% CO + 10% helium
- Quantity of gas adjusted to roughly TV of pt
- Gas sampled & DLCO calculated in similar way to single breath
- Steady state method*:
- Breathe in controlled gas mixture with 0.3% CO
- Exhaled gas collected
- After a period of breathing (long enough for steady state to be established), exhaled gas is analysed
- CO delivery and exhaled gas volume known –> calculate CO uptake
* Not really used in clinical practice
Describe the carriage of oxygen in blood
Oxygen storage
- We contain ~1.55L of oxygen in our body
- ~850mL in blood
- 20.1mL/dL in Hb
- 0.3mL/dL dissolved
- ~200mL in myoglobin
- ~450mL in FRC (this is why pre-oxygenation is a great O2 reserve for apnoeic ventilation)
- ~50mL in tissues
Oxygen transport
Transported in blood via:
- Dissolved oxygen with a partial pressure at pulmonary capillaries of 100mmHg
- Haemoglobin
- Tetramer with 2x alpha & 2x beta subunits
- Each subunit has a heme porphyrin (with central Fe atom)
- Central Fe has total 6 binding sites - 4 taken up by purines, one by histidine & one spot available for molecular O2
- Binding of O2 results in conformational change to the rest of the haemoglobin molecule which increases the affinity of further O2 binding
- Results in O2-Hb dissociation curve to be sigmoid
- Oxygen combining capacity of Hb is 1.306 ml of O2/ gm of Hb when fully saturated (1.34 in CaO2 equation)
Oxygen delivery/oxygen flux:
- Oxygen flux is the amount of oxygen delivered to tissues every minute (sometimes called global oxygen delivery (DO2))
- Equal to cardiac output multiplied by oxygen content (CO x CaCO2)
Oxygen consumption:
- Tissues consume approximately 250mL O2/min (VO2)
- Oxygen extraction ratio (O2ER) = VO2/DO2 = (SaO2 - SvO2)/SaO2 = 25% in normal adult at rest.
- O2ER can increase to 70% in states of exercise
Explain the oxyhaemoglobin dissociation curve and factors that may alter it including the Bohr and Double Bohr effect
The oxygen dissociation curve is a graph displaying the relative association between partial pressure of oxygen and the affinity of oxygen to haemoglobin (i.e. oxyhaemoglobin saturation)
- The flat upper plateau decreases variability in in blood oxygen content even with large changes of PaO2
- The steep lower part allows the increased release of oxygen from haemoglobin with only a small change in PaO2
- P50: shows the partial pressure of O2 where haemoglobin is 50% saturated (P50 = 26.6mmHg)
- The properties of haemoglobin that affect the shape of the curve:
- Hb is a tetramer with 2x alpha & 2x beta subunit
- Each subunit has a heme porphyrin (with central Fe atom)
- Central Fe has total 6 binding sites - 4 taken up by purines, one by histidine & one spot available for molecular O2
- The Tensed state “T” is the deoxygenated form with 0 O2 molecule & “R” state is the oxygenated state with 4 O2 molecules
- Binding of O2 results in conformational change to the rest of the haemoglobin molecule which increases the affinity of further O2 binding
- This is called positive cooperativity and results in O2-Hb dissociation curve to be sigmoid
Factors affecting it:
Conditions which shift the curve to the right:
- Increased PCO2, temperature, 2,3-DPG
- Presence of sulfhaemoglobin
- Decreased pH
The above reduces affinity for O2 to Hb and oxygen offloading at the tissues
Conditions which shift the curve to the left:
- PCO2, temperature, 2,3-DPG, pH and carbon monoxide (CO)
- Unusual haemoglobin species shift the curve to the left (methaemoglobin, carboxyhaemoglobin, foetal haemoglobin)
The above increases the affinity of O2 to Hb and reducing offloading
[Note: 2,3-DPG: promotes the offloading of oxygen. It is produced from a side reaction of glycolysis and is upregulated in anaemia (due to relative tissue hypoxia), at altitude and is reduced in stored blood]
Bohr effect: reduced affinity of O2 for Hb when pH is low, and vice versa when high à promotes offloading
Double Bohr effect: aids to offload O2 to foetus à the curve shifts to the right in the mother and to the left in the foetus facilitating transfer across placental circulation to foetus

Describe the carbon dioxide carriage in blood including the Haldane effect and the chloride shift
CO2 is an end product of aerobic metabolism, produced by all mitochondria and is produced at a rate of ~200mL/min
Carriage of CO2 in blood:
- Bicarbonate (~ 70-90%). As intracellular bicarb concentration increases, bicarb is exchanged for chloride (which causes the chloride shift - venous Cl is lower than arterial)
- Bicarb is formed by the following sequence:
- CO2 + H2O (catalysed by CA) H2CO3 H+ + HCO3-
- The first part is very slow in plasma, but fast in RBCs due to carbonic anhydrase. The second part is fast physiologically
- Carbamino compounds eg. Hb (10-20%)
- The dissociation of O2 causes a conformational change (movement of N-terminus) which increases the affinity for CO2 (binds at N-terminus of alpha chain)
- Deoxy-Hb is 2-3x better at forming carbamino compound than oxy-Hb (Haldane effect - the physicochemical phenomenon which describes the increased capacity of blood to carry CO2 under conditions of decreased haemoglobin saturation)
- Dissolved CO2 (10%)
- CO2 is 20-24x more soluble than O2
- Henry’s law - the amount of dissolved gas in a liquid is proportional to its partial pressure above the liquid
- Carbonic acid (<1%)
* H2CO3 - at physiological pH, carbonic acid is ~96% dissolved - this is present in high concentrations in erythrocytes and pulmonary capillaries
Explain the carbon dioxide dissociation curve
The CO2 dissociation curve describes the relationship between PCO2 and total CO2 concentration of blood
- The lower the Hb saturation with O2, the higher the total CO2 concentration for every pCO2. This is because of the increased affinity of Hb for CO2 when Hb is deoxygenated (Haldane effect)
- The arterial point corresponds to the CO2 content of arterial blood PCO2 is 40mmHg and CO2 content is 480ml/L. The total CO2 content in venous blood at the same PCO2 would be ~ 500mL/L (mostly due to Haldane effect)
- The mixed venous point corresponds to the CO2 content of mixed venous blood, where PCO2 is 46mmHg and CO2 content is 520mL/L. Due to Haldane effect, if this blood was ‘arterialised’ by addition of O2 while total CO2 content remained the same, the extra CO2 liberated by the oxygenation of Hb would produce an increase in the PCO2
The ‘physiological CO2 dissociation curve’ refers to the line that is draw between the arterial point and mixed venous point. This describes the change in CO2 in blood as it travels in the circulation (see attached image)

Describe the oxygen and carbon dioxide stores in the body
The human body is approximately 61-64% oxygen by weight
Oxygen stores in the body that are accessible:
- Dissolved oxygen as molecules of O2 in blood and generally in body water
- 50mL
- Bound gas - Hb/Mb/Other
- ~850mL in Hb
- ~200mL in Mb
- Gas in cavities (eg. FRC)
- ~450mL
Carbon dioxide stores:
- Bone stores
- 30% bicarbonate; 70% carbonate
- Part of bone matrix & forms 15% of total daily CO2 production
- About 9% of bone stores are accessible to assist with buffering (up to 14L or 200mL/kg)
- Dissolved carbon dioxide
- 3L available for immediate use in buffering
- 80-90% stored in the form of bicarbonate anions (HCO3-)
- 5-10% present as unchanged gas, dissolved in the water of ECF (predominantly blood)
- 5-10% as carbamino compounds
- 2-5% available as free gas in alveolar gas mixture
- Miniscule amount as carbonic acid
Describe physiology and consequences of foetal haemoglobin
Fetal blood:
- Fetal haemoglobin Made up of 2α + 2gamma subunits (as opposed to 2α+ 2ß)
- The subunits don’t allow for binding of 2-3DPG, which has the effect of shifting the O2-HbF dissociation curve to the left (compared to adult)
- P50 = 19
- Fetal blood has a higher haematocrit and overall higher oxygen content (210mL/L vs 130mL/L in adult blood at PaO2 100mmHg)
Physiological consequences in the maternal/fetal circulations:
- Double Bohr effect:
- Increased CO2 + decreased pH in fetal circulation will favour the offloading of oxygen from maternal Hb (first effect)
- The decreased CO2 (due to oxygenation of HbF) increases the affinity of HbF for O2 (second effect)
- Ie maternal O2-Hb dissociation curve shifts to right + fetal O2-HbF dissociation shifts to left
- Double Haldane effect:
- As it becomes more oxygenated, foetal haemoglobin releases CO2 (first effect)
- Deoxygenated maternal blood has increased affinity for fetal CO2 (second effect)
Explain the similarities and differences between myoglobin and adult haemoglobin (60% of marks) and their physiologic relevance (40% of marks)
(Exam length question)
See image

Describe physiology and consequences of abnormal haemoglobin
(Own question)
Methaemoglobin
Methaemoglobin is a state of haemoglobin in which the iron in the heme group is in its ferric (Fe3+) state instead of ferrous (Fe2+). Ie it is oxidised haemoglobin. (usually, O2 binding to heme forms Fe+++O2- and O2 dissociating will will leave Fe++. But occasionally, superoxide (O2-) will dissociate to leave Fe+++)
- Methaemoglobin encompasses any of the subunits of haemoglobin being oxidised
- Darling-Roughton effect - the loss of one haem interferes with cooperativity of the haemoglobin
- Oxidation of heme results in the Hb molecule having a net positive charge compared to normal Hb and therefore has greater affinity for anions eg. Cyanide, flouride, chloride
- Physiological production - normal autooxidation of Hb occurs 0.5-3% of total Hb/day
- Acidosis can cause increased production
- Cytochrome b5, cytochrome b5 reductase 3 usually responsible for clearing metHb
Carboxyhaemoglobin
- Carboxyhaemoglobin is Hb that has bound to CO instead of O2.
- This form of Hb also interferes with cooperativity - dissociation curve is no long sigmoid
- Decreases oxygen carrying capacity of blood
- P50 - 12mmHg
Others
- HbA2
- This is found in thalassemia, sickle cell anaemic, hyperthyroidism
- 2 alpha + 2 delta subunits
- Poor at oxygen transfer
- Sickle cell Hb
- Mutation of b chain
- Shifts curve to the left
- Thalassaemia
- Equal quantities of a and b chains but excessive production and one may predominate
- Get abnormal amounts of HbA2 and HbF
- Leads to haemolysis and anaemia
Describe the Helium dilution method of measuring FRC
(own)
Tracer gas dilution
* ○ Closed circuit system where spirometer is filled with a mixture of He + O2 (conc and volume of spirometer gas is known)
* ○ The subject breathes in the mixture starting from FRC. They inhale this volume and it is distributed widely and evenly in their lung volume
* ○ There is equilibration of helium throughout the spirometer and lungs & the new concentration of helium in the spirometer is measured
* ○ From this concentration, the volume of distribution of that gas can be calculated (ie. Intrathoracic volume):
C1 × V1= C2 ×(V1+ V2 )
C1 = Initial tracer concentration V1 = Initial volume C2 = tracer concentration after equilibration V2 = FRC
Note:
* In practice O2 must be added to make up for that absorbed by the patient and CO2 must be absorbed.
* This technique only measures gas in alveoli that communicate with the mouth. Gas trapped behind closed airways is not measured

Describe how body plethysmography can be used to measure FRC
(own)
Body plethysmography
- This is a method that uses Boyle’s law to measure lung volumes. The experiment is conducted as follows:
- The patient and equipment are situated in a tight box with known volume and pressure
- The patient breathes through a tube connected to the outside & the tube is clamped off at the start of the experiment, when the patient is at FRC
- The patient attempts to breathe in through the tube (No air flows as the tube is clamped) & the negative pressure generated to draw in that breath is measured at the mouth (Pm)
- Given that all volumes and pressures can be measured, and the only unknown is the volume inside the chest, the volume can be calculated using the equations below (see image)

Describe spirometry and the normal values for pulmonary function tests
(not exam length)
Spirometry:
- Measurement of the relationship of expired volume over time, or expiratory flow, typically by a maximally forceful expiration from TLC down to RV
- Typically performed after lung volume measurements - measures Forced Expiratory Volume (FEV)
- Forced vital capacity (FVC) = time and volume for forced expulsion of VC (from TLC)
- FEV1 = volume of air that can expired in one second
- FEV1/FVC ratio is used to measure whether defects are restrictive or obstructive
- Restrictive lung diseases may have preserved FEV1/FVC ratio, but TLC will be reduced
- Obstructive lung diseases will have decreased FEV1/FVC ratios
- Normal values:
- FVC > 80% predicted
- FEV1 > 80% predicted
- F25/75 (this measure flow at mid exhalation) > 80% predicted
- If all other values are normal, and this is reduced, this indicates small airways disease (usually seen in cigarette smokers as precursor to COPD)
- PEF should be at least as large as the lesser of FVC + FEV1
- Lower values may indicate weakness, central obstruction (eg. Tracheal stenosis) or sub-maximal effort
- FEV1/FVC > 0.70 (this is cut off for COPD diagnosis)
- Note: high values for TLC or RV are only significant if there is a ventilatory defect
- DLCO >80% is normal
Describe the carbon dioxide and oxygen response curves and how these may be used to assess the control of breathing
(exam length question, never before asked)
The carbon dioxide and oxygen response curves describe what happens to minute ventilation with changes in partial pressures of these gases in the circulation
- As PaCO2 increases, minute ventilation increases in a linear and reproducible way (given PaO2 remains constant)
- Minute volume should increase by 3L/min for every 1mmHg PaCO2
- As PaO2 decreases, minute ventilation increases (in a non-linear way, given PaCO2 is constant)
- MV should triple when PaO2 drops to 40mmHg/sats 60%
If other pulmonary function tests are normal, but a patient remains hypoxic or hypercapnoeic, the following tests can be done to asses control of breathing:
Hypoxic challenge:
- Patient is given a hypoxic gas mixture to breathe in with constant CO2 (usually have a closed circuit with a CO2 scrubber)
- With each breath, the content of O2 will decrease
- The patient’s MV is expected to increase - increased RR, or tidal volume or both
- This is mainly a test of peripheral chemoreceptor function
- Reasons for abnormal response:
- Intrinsic chemoreceptor issue
- Problem with central integration
- Poor sensitivity to hypoxia
- Chronic exposure to: high altitude; hypoxia due to disease; high oxygen demand (endurance athletes)
- Age-related desensitisation
Hypercapnoeic challenge:
- Similar to above, but the circuit is filled with 100% FiO2 + CO2 scrubber removed
- Therefore patient is breathing in an increasingly hypercapoeic gas mixture (overall hyperoxic too)
- MV should increase in response to hypercapnoea
- This is a test of central chemoreceptos
- Reasons for abnormal response:
- COPD, OSA, OHS
- Metabolic alkalosis
- Elderly patients
- Endurance athletes
- Hypothyroidism
Mouth Occlusion Pressure:
- Measures the maximum pressure which is generated over the period of a 100-ms occlusion of the airway (occlusion so brief that no voluntary response is possible)
- Pressure represents pressure generated by inspiratory muscles

Describe the physiological consequences of intermittent positive pressure ventilation and positive end-expiratory pressure
(exam length question)
PEEP is a residual pressure above atmospheric maintained at the airway opening at the end of expiration
Respiratory:
- Increases FRC
- Increase alveolar recruitment –> improved V/Q matching
- Increases total gas exchange interface
- Increases compliance (usually easier to increase the volume of already inflated alveoli)
- Decreases WOB
- PEEP may distribute lung water out of the interstitium
- Excessive positive pressure leads to
- Overdistention + Lung injury
- Worsening of V/Q matching
- “biotrauma” (cytokine leak + extrapulmonary organ dysfunction)
Cardiovascular:
- RV + pulmonary circulation:
- Increase intrathoracic pressure is transmitted to central veins + RA –> decreased RV preload
- Increases PVR –> Increases afterload
- This has the net effect of decreasing RV stroke volume
- LV + systemic circulation
- Decreases preload (decreased pulmonary venous pressure)
- Decreases afterload - (decrease in LV end-systolic transmural pressure + increased gradient between intrathoracic aorta and extrathoracic systemic circuit)
- Overall, increased PEEP causes decreased CO + decreased myocardial O2 demand
Other organ systems:
- Neuro: raised ICP if PEEP is very high
- Endocrine: sodium retention due to decreased ANP release and aldosterone secretion (will also get water retention)
- Renal: decreased renal perfusion and GFR (due to decreased CO + increased renal venous pressure)
- GIT:
- Decreased hepatic perfusion + decreased metabolic clearance of drugs
- Decreased splanchnic perfusion - decreased intestinal motility + poor gastric emptying
- Decreased gastric perfusion - increases risk of stress ulceration
- Immune: neutrophil retention in pulmonary capillaries + impaired lymphatic drainage from lungs
Outline the non-ventilatory functions of the lungs
(Exam length question)
Mnemonic: DRIFT(s) ME
Drugs (pharmacological)
- Administration (inhalational drugs) + elimination (eg. Volatiles)
- Sequestration of drugs (ie “first pass uptake”) - eg. Fentanyl, lignocaine + propofol (later releases these into arterial circulation as a 2nd peak)
Reservoir
- Of blood
- Can accommodate up to 450mL blood volume by recruitment + distention
- Mobilisation of this blood occurs with SNS stimulation (constricts pulmonary vessels) + raised intrathoracic pressure
- Of gas
- Volume of air at FRC is an oxygen reservoir
Immunological/defence
- Physical
- Upper airways - nasal hairs, mucous, mucociliary escalator
- Airway reflexes - coughing + sneezing
- Cellular
- Alveolar macrophages + lung neutrophils
- Other
- Lymphatics
- Secretory IgA
- Direct antibacterial action of surfactant
Filtration
- Of blood
- Due to narrow capillary bed (7um). Particles >10um are prevented from entering the systemic circulation
- VTE - trapped in pulmonary circulation and broken down by intrinsic fibrinolytic activity of the lungs (heparin-containing mast cells within lung interstitium)
- Microbes from venous circulation filtered and prevented from entering the arterial circulation
- Due to narrow capillary bed (7um). Particles >10um are prevented from entering the systemic circulation
- Of gas
- Particles are deposited into the lung by interception (fibres/close contact), impaction (particles don’t turn with air), sedimentation (gravitational forces) and diffusion (random motion of particles)
Thermoregulation (and water)
- Heat is dissipated through respiration. Inspired air equilibrates to body temperature & is expired as warm air. Mostly by upper airway
- We lose about 230kcals/day by this route (about 12% basal heat loss) (This is the energy needed for vaporisation)
- Extravascular water content of lungs 300-400mL normal - can increase in disease states. “Insensible water losses” of ~400mLs
Speech
Metabolic/Endocrine
- Metabolism of vasoactive substances
- Activation of angiotesnsin I –> II (pulmonary capillaries have highest ACE)
- Inactivation of bradykinin (ACE deactivates 80% of bradykinin)
- Uptake and degradation of 5-HT (100%) and Nad (30%; via COMT + MAO)
- Enzymatic inactivation of PGE-1 & -2, PGF-2α
- Protein synthesis (collagen + elastin to maintain lung structure)
- Carbohydrate metabolism
- Removal of proteases (via α-1 antitrypsin
- Production of surfactant
Describe the adverse effects of hyperoxia
(own question)
Hyperoxia
- Normobaric hyperoxia
- Airway: Tracheobronchitis + mucositis
- Lung: Alveolar (pulmonary) toxicity, pulmonary vasodilation, decreased respiratory drive
- Absorption atelectasis - due to the low solubility of N2, it acts as a ‘splint’ to keep alveoli open
- Delivering 100% FiO2 washes out O2, which dissolves much more readily into the circulation
- Absorption atelectasis - due to the low solubility of N2, it acts as a ‘splint’ to keep alveoli open
- Gas carriage: reverse Haldane effect (release of CO2 from Hb), increased clearance of CO, increased denitrogenation of gas cavities (eg. Pneumothorax)
- Cardiovascular system: vasoconstriction due to accelerated oxidative degradation of NO in endothelium
- CNS: Mild euphoria, decreased cerebral blood flow
- Metabolic: Increased free radical production
- Bone marrow + immune system: impaired eythropoiesis, immunosuppresssion, impaired reproduction of anaerobes
- Hyperbaric hyperoxia
- Airway: Mucosal damage worsens in proportion to duration of exposure and increasing PO2
- Lung: Accelerated alveolar toxicity, transitioning to ARDS
- Gas transport: O2 contributes significantly to total gas transport (may not need Hb!)
- Cardiovascular system: Hypertension, with reflex bradycardia + decreased CO
- CNS: seizures, (reversible) myopia, decreased ICP and cerebral blood flow
- Endocrine/electrolytes: BSL + Na decreases, K increases
- O2 toxicity
- CNS stimulation
- Another form of O2 toxicity - can lead to convulsions when PO2 considerably exceeds 760mmHg
- Likelihood of developing convulsions depends on the inspired PO2 and the duration of exposure (at 4 atm, convulsions can occur at 30min)
- O2 concentration should be progressively reduced for deep dives to avoid toxic effects
- CNS stimulation
- Therapeutics - Hyperbaric oxygen therapy indications:
- CO poisoning - raising inspired PO2 to 3 atm, dissolved O2 can be increased to 6mL/100mL & needs of the tissues can be met without functioning Hb. Similar situation for severe anaemia
- Necrotising fasciitis - organism cannot live in high PO2 environment
- Decompression sickness
Describe the different types of hypoxia and the effects it has on end organs
(own question)
Background - hypoxia
- Definition: presence of tissue pO2 levels which are low enough to have adverse effects on tissue function
- Can be classified into 4 different types:
- Hypoxic/hypoxaemic hypoxia - hypoxaemia is reduced arterial pO2. Most common form of hypoxia. Caused by V/Q mismatch, disruptions to the gas exchange interface or neurologically mediated ventilatory failure
- Anaemic hypoxia - usually not severe at rest, however pts with anaemia may become short of breath on exercise due to inability to adequately transport oxygen to tissues during exercise. CO poisoning is a form of anaemic hypoxia (as there is less Hb with O2 carrying capacity)
- Ischaemic/stagnant hypoxia - occurs in slow/low flow states (eg. Shock)
- Histotoxic hypoxia - hypoxia due to inhibition of tissue oxidative process - eg. Cyanide inhibits cytochrome oxidase
Effects of hypoxaemia
- Cellular effects:
- Hypoxia causes production of hypoxia-inducible factors (HIFs). These are made up of α and β subunits. α subunits are usually ubiquinated and destroyed, but in hypoxic cells, they dimerise with β subunits and the dimers activate genes for proteins such as angiogenic factors, erythropoeitin, & proteins that alter immune cell function (increased pro-inflammatory cytokines, chemotaxis, IL-8 & TNF-α production, Stimulation of VEGF)
- Brain:
- Sudden drop in inspired pO2 to <20mmHg will cause loss of consciousness in 10-20s & death in 4-5min (eg. Sudden loss of cabin pressure in plane at 16000m)
- Less severe hypoxia causes symptoms similar to EtOH intoxication: impaired judgement, drowsiness, dulled pain sensibility
- Signs include tachycardia, and when severe, hypertension. Also have increased respiratory rate
- Above slide into coma/seizures
- Respiratory
- May get cough suppression, airway reflexes remain relatively intact
- Respiratory drive - decreasing PaO2 causes increase in minute ventilation
- Gas exchange - if there is a very low A-a gradient, gas exchange may not occur. Additionally, hypoxia damages alveolar cells (which derive their oxygen from the ambient alveolar air - so if the O2 content is low, alveolar cells will suffer hypoxic damage and gas exchange will be impaired)
- Cardiovascular
- Hypoxic pulmonary vasoconstriction in pulmonary circulation
- Regional hypoxic vasodilation in systemic circulation - cerebral & coronary artery vasodilation but systemic hypoxic vasoconstriction + hypertension due to sympathetic overactivity driven by activated carotid and aortic chemoreceptors
- Bradycardia is a late sign of hypoxia
- Acid-base changes - initial respiratory alkalosis (hypoxic drive), follow by metabolic acidosis (anaerobic metabolism - lactate mediated)
- Renal - kidneys tolerate hypoxia fairly well. Severe hypoxia possibly causes ATN
- Liver - initially decreases some metabolic processes in order to decrease its own oxygen consumption, then releases lots of glucose to produce more ATP needed for anaerobic metabolism. It can gradually extract more and more O2 from perfusing blood so that ER = 100%
Describe the effects of hypo- and hyper- capnia
(Own question)
Hypocapnia
- Cerebral blood flow can decrease due to direct constrictor effect of hypocapnia. This can cause light-headedness, dizziness & paraesthesias
- Ionised calcium levels fall
- Renal acid secretion is inhibited
Hypercapnia
- Difficult to separate effects of hypercapnia from those of acidosis
- Most textbooks say that hypercapnia depresses and ultimately abolishes the activity of respiratory control centres however unclear mechanism - may be due to its anaesthetic effects (?neuronal intracellular acidosis)
- Causes bronchodilation
- Right shift of oxygen-haemoglobin dissociation curve
- Sympathetic stimulation and catecholamine excess
- Causes vasodilation - worry about vasodilation of cerebral arteries in TBI
Explain the effect of changes in posture on ventilatory function
(own question)
Compliance:
- Only about an 11% change in ventilatory function based on posture
- Highest compliance when sitting in upright positions (with 30o head up best) and worse in lateral position
- Prone positioning can help to decrease the pleural pressure gradient between lung units (makes them more homogenous) to decrease the VILI
Resistance:
- Overall respiratory resistance is highest in supine position and lowest in lateral
- Chest wall resistance is highest in prone position while lung resistance is highest in supine/lateral
Respiratory volumes:
- FRC decreases in the supine position mainly due to increased chest wall resistance (from abdomen)
- FRC either stays the same or increases in prone positioning (due to increase ERV)
Briefly outline how the humidity of air is altered during inspiration and expiration by the respiratory tract and the explain the importance of humidification
(own question)
- Inspired gas has a water content of around 10g/kg (50% humidity, 22OC)
- This gas is heated and humidified in the URT. Turbulence in the nasal cavity increases evaporative heat exchange between the air and mucosa & the inspired air is isothermic around 5cm beyond the carina (isothermic saturation boundary)
- Mucosa transfers heat + water to air via the process of evaporation (mucosa is cooled)
- On expiration, some of this heat + moisture is reclaimed by condensation
- In hot environments, humidity cannot be reclaimed + water loss increases
- Alveolar gas has a water content of 47g/kg (100% humidity, 37%) - this has an effect on the relative partial pressures of different alveolar gases and may have an impact on gas exchange
- Consequences of impaired humidification
- Local dehydration impairs bronchial immunity & cilial paralysis/reduced rates of mucous flow occur below 40% humidity
- Effects of intubation
- ETT will bypass upper airway (and therefore most of the intrinsic humidification mechanisms of the airway)
- Can have increased water losses (loss of reclaimation of heat/moisture)
Explain the pathways and importance of the cough reflex
(exam length question)
Purpose and importance of cough:
- Protective function - defence against foreign material in the airway
- Pathological consequences - damage to mucosa with persistent/unproductive cough
- Diagnostic purposes - evidence of intact medullary function
Stimulus
- Exogenous/endogenous stimuli to the airways
- Chemical/biological
- Acids (gastric contents)
- Biological pathogens
- Mediators associated with inflammation
- Mechanical
- Aspiration of liquids
- Solids
- Chemical/biological
Afferent
- Rapidly adapting receptors
- Respond to dynamic lung inflation, bronchospasm or lung collapse
- Sporadically active throughout the respiratory cycle
- Mechanical stimuli
- Slowly adapting stretch receptors
- Sensitive to mechanical forces
- Participate in the Hering-Breuer reflex
- C-fibres
- Nociceptors - similar to those in skin
- Respond to noxious chemical and mechanical stimuli
- Acids, pathogens,inflammatory
- These receptors connect to the medullary control centre by vagus nerve fibres
Central control
- Located at caudal 2/3rds of NTS
- NTS –> central control (medullary)
Efferent pathway of cough reflex
- To diaphragm - via phrenic nerve
- To Abdo muscles - via spinal motor nerves
- To larynx - via laryngeal branches of vagus, from nucleus ambiguus
Phases of the cough reflex:
- Sensory phase - afferent fibres conduct mechanoreceptor + chemoreceptor stimuli to central integrator in medulla + a cough reflex is triggered
- Inspiratory phase: glottis opens and a deep breath is inhaled
- Compressive phase: glottis closes and expiratory muscles forcibly contract - ITP may >100cmH2O
- Expulsive phase: glottis opens and rapid airflow begins. Bronchial tissue oscillate due to rapid turbulent flow, which loosens secretions
Describe the pharmacology of ß-agonists used in the treatment of asthma
- Chemistry: similar in structure to NE + adrenaline. Terminal amine group is modified to confer β2-selectivity
- Preparation: Inhaled via MDI, or nebulised. Salbutamol available in IV + PO (liquid)
- Dose/indication:
- SABAs:
- Symptom relief of asthma + COPD
- Prevention of exercise-induced bronchoconstriction
- Note: IV salbutamol used in threatened/preterm labour - relaxes uterine smooth muscle via uterine β2 receptors
- LABAs:
- Maintenance treatment of asthma in patients receiving inhaled or oral corticosteroids (LABA rarely/never used as monotherapy)
- COPD
- SABAs:
- Kinetics
- Absorption: terbutaline 33-50% absorption
- Distribution:
- SABAs: protein binding 10% salbutamol; terbutaline 25%
- LABAs: salmeterol 96%, formoterol ~64%, indacaterol 95%, 60% olodaterol
- Metabolism:
- SABA: salbutamol & terbutaline: hepatic to inactive sulfate; levalbuterol metabolised in GIT
- LABA: salmeterol & indacaterol hepatic - hydroxylated; formoterol & olodaterol hepatic - glucuronidation
- Excretion: mostly excreted in urine; salmeterol & indacaterol in faeces; olodaterol both
- Dynamics
- MoA
- Activation of Gs protein coupled receptor pathway (adenylate cyclase –> cAMP –> PKA –> phosphorylative events) - leading to bronchial smooth muscle relaxation
- Lowers intracellular Ca2+ concentration
- Acute inhibition of PLC-IP3 pathway and its mobilisation of cellular Ca2+
- Act as functional antagonists (reverse bronchoconstriction irrespective of the contractile agent
- Activation of Gs protein coupled receptor pathway (adenylate cyclase –> cAMP –> PKA –> phosphorylative events) - leading to bronchial smooth muscle relaxation
- Effects/adverse
- Resp: bronchodilation
- CVS: sometimes induces tachycardia in high doses
- Endo: Hypokalaemia (effect on Na/K ATPase)
- Other
- MoA
Describe the pharmacology of anti-muscarinic drugs used in the treatment of asthma
- Chemistry - Atropine + scopolamine are naturally occurring - esters formed by combination of aromatic acid, tropic acid + complex organic bases. All other antimuscarinics are derived from these
- Ipratropium and tiotropium are quaternary ammonium compounds
- Preparation - ipratropium - solution or powder for inhalation; tiotropium powder only
- Dose/Indication - COPD, asthma
- Kinetics
- Absorption - Ipratropium/tiotropium (quaternary muscarinic receptor antagonists) not well absorbed via GIT.
- Distribution - Ipratropium 10-15% of dose reaches lower airways (~90% of inhaled amount is swallowed)
- Metabolism - ipra –> partially metabolised to inactive ester hydrolysis products
- Excretion - urine + faeces; 2 hrs half life
- Dynamics
- MoA
- Competitive antagonists of Ach binding to muscarinic cholinergic receptors - block the effects of endogenous ACh at muscarinic receptors
- Ipratropium is non selective (therefore also inhibits M2 receptors at presynaptic neurons, which provides ACh- mediated inhibition of ACh release - ie. It stops negative feedback, resulting in further ACh release into the synapse. This might work to oppose the muscarinic blockade at the postsynaptic neuron (conversely, tiotropium has lower affinity for M2)
- Effects/adverse effects
- CNS - drowsiness, dizziness, confusion
- CVS - tachycardia
- GI - constipation
- MoA
- Other
Describe the pharmacology of methylxanthines used in the treatment of asthma
- Chemistry - (theophylline + aminophylline) are methylxanthines similar in structure to common dietary xanthines
- Aminophylline is a complex of theophylline and ethylenediamine
- Chemically similar to caffeine
- Preparation: PO/PR/IV formulations; Theophylline available in PO form only
- Dose/Indication: Acute severe asthma, not responding to other therapies
- Aminophylline: Loading IV 5mg/kg (rate <25mg/min); maintenance IV 0.5mg/kg/hr (in patients not previously treated with theophylline)
- Theophylline used in maintenance treatment rarely. Initial 300mg daily
- Kinetics
- Absorption: Rapidly absorbed. Aminophylline has high PO bioavailability (88-96%)
- Distribution: 50-60% protein bound (albumin), Vd = ~0.5L
- Metabolism - metabolised in liver - demethylation + hydroxylation; forms active metabolites
- Excretion - saturation of metabolic pathways occurs near therapeutic range - may have zero-order + first order kinetics. Under first-order, half-life is 8hrs
- Dynamics
- MoA - unclear but several proposed MoA. Onset 1-2hrs PO; 30mins if IV
- Inhibition of phosphodiesterases - theophylline is a non-selective PDE inhibitor (but inhibition is minimal within the ‘therapeutic range’). PDE inhibition likely explains bronchodilator effect of theophylline, but does not account for clinical benefit in asthma seen at the low concentrations it is used in
- Adenosine receptor antagonism - may reduce inflammation by blocking A2B Receptor on mast cells (activated by adenosine in asthmatics). This mechanism accounts for serious side effects (blockage of cardiac + central A1 receptors)
- May promote apoptosis of eosinophils + neutrophils (by A2A antagonism) + T lymphocytes (PDE inhibition)
- Histone deacetylase (HDAC2) activation enhances anti-inflammatory effects of corticosteroids. This happens at the low concs used in asthma
- Other - many other effects
- Non-bronchodilator effects - the clinical benefits from theophylline use occur at plasma concs low enough to explained by bronchodilator action. Increasing evidence that theophylline has anti-inflammatory effects in asthma
- Effect/side effects
- CNS: headache, nausea, vomiting (due to inhibition of PDE4); at high concs, may have seizures from central A1 receptor antagonism
- CVS: in high concentrations, cardiac arrhythmias may occur due to PDE3 inhibition & antagonism of cardiac A1 receptors
- GI: Increased acid secretion (PDE inhibition), abdo discomfort
- Renal: diuresis (Adenosine A1 inhibition)
- MoA - unclear but several proposed MoA. Onset 1-2hrs PO; 30mins if IV
- Other
- Large variations in clearance - dosage needs to be individualised by measurement of plasma concentrations
- Plasma monitoring important. Target theophylline conc 5-10mg/L
- May have a role in prevention of progression of COPD due to anti-inflammatory effects
Outline the pharmacology of drugs used to treat acute pulmonary hypertension
Nitric oxide:
Pharmaceutics
- Class/chemistry: selective pulmonary vasodilator. Inorganic gas
- Preparation: In aluminium cylinders containing 100/800ppm of NO & N2. Pure NO is toxic and corrosive
- Administration/dose: 5-20ppm dosing - injected into patient limb of inspiratory circuit or via continuous flow
- Indication: pulmonary hypertension
Pharmacokinetics
- Absorption: Highly lipid soluble and diffuses freely across all membranes
- Distribution: VOD potentially very large
- Metabolism: Following inhalation, NO combines with oxyhaemoglobin that is 60-100% saturated, producing methaemoglobin and nitrate. During the first 8hrs of NO exposure, methaemoglobin concentrations increase
- Excretion: NO has a half-life of <5s. Main metabolite is nitrate (70%) which is renally excreted
Pharmacodynamics
- Mechanism of action/adverse effects - NO diffuses into vascular smooth muscle and stimulates guanylate cyclase which catalyses the formation of cGMP, which activates a phosphorylation cascade which leads to smooth muscle relaxation and vasodilation
- Resp - inhibits hypoxic pulmonary vasoconstriction & preferentially increases blood flow through well-ventilated areas of lung
- Toxicity - exposure to 500-2000ppm results in methaemaglobinaemia and pulmonary oedema. Contamination by NO2 can lead to pneumonitis and pulmonary oedema
- CVS - NO is avidly bound to Hb and inactivated before reaching the systemic circulation
- Resp - inhibits hypoxic pulmonary vasoconstriction & preferentially increases blood flow through well-ventilated areas of lung
- Other
Prostacyclin (Epoprostenol = synthetic version)
Pharmaceutics
- Class/chemistry: Inhaled pulmonary vasodilator. Epoprostenol is the synthetic analogue of naturally occurring prostacyclin (PGI2)
- Preparation: as vials containing 500 micrograms of freeze-dried epoprostenol sodium - to be diluted in sodium chloride
- Administration/dose: Can be IV, but usually nebulised as a solution with a glycine buffer
- Indication
Pharmacokinetics
- Absorption: rapidly absorbed into pulmonary circulation via the lungs
- Distribution: 0.35L/kg
- Metabolism: Degrades spontaneously as well as enzymatically into about sixteen major and minor metabolites
- Excretion: Half life ~ 6 minutes; vasodilation is short lived while platelet inhibition lasts up to 2 hrs
Pharmacodynamics
- Mechanism of action - stimulates adenylate cyclase, leading to an increase in cAMP concentration within platelets, which leads to inhibition of platelet phospholipase and COX, and ultimately of platelet aggregation
- Resp: decrease in PVR and interferes with hypoxic pulmonary vasoconstriction
- CVS: Relaxation of vascular smooth muscle, leading to decrease in systemic vascular resistance
- CNS: Cerebral vasodilation - increased cerebral blood flow
- Haem: powerful platelet inhibitor - bleeding time may double with higher doses
- Other: extends filter life during RRT
Describe the principles of pulse and tissue oximetry, co-oximetry including calibration, sources of errors and limitations.
Biological variable being tested:
- Percentage saturation of haemoglobin
Sensor:
- 2 LED emitting light with wavelengths 660nm (red light) & 940nm (infrared)
- The lights are turned on sequentially, then both turned off. Light sources flicker with frequency 300-900Hz
- While each light is on, the sensor (2 photodiodes) detects how much of the emitted light reaches it. Deoxyhaemoglobin absorbs more red light, while oxyhaemoglobin absorbs more infrared light
- The sensor is converted to an electrical signal which goes to the integrator
Integrator:
- Beer’s Law - the absorption/attenuation of light as it passes through a substance is proportional to the concentration of a substance
- Lambert’s Law - the absorption/attenuation of light as it passes through a substance is proportional to the distance the signal has to travel
- With each cardiac cycle, the diameter of the blood vessels supplying the fingers will increase with the cardiac output, thus increasing optical path length (which will increase absorbance)
- Numerically, Beer-Lambert Law can be represented by
Where:
A = absorbance
l = optical path length
- How the signal is modified:
- Calibration - The variance in electrical signal can be separated into pulsatile and non-pulsatile components (by assessing AC vs DC current) & an R value is calculated (AC660nm/DC660nm divided by AC940nm/DC940nm).
- R values are calculated from healthy volunteers and graphed for Sats to 75%. Below these Sats, R value is extrapolated
- Non-pulsatile signal includes non-pulsatile arterial, venous and tissue absorbance
- Removal of ambient light - when both LEDs are off, the amount of ambient light of wavelengths 660nm and 940nm is detected by the sensors and removed from signal analysis
- Removal of movement artifact - Once computer has removed the non-pulsatile arterial & ambient light signals, the computer takes the pulsatile part of the signal and amplifies it - takes average over few seconds to remove movement artifect
- Calibration - The variance in electrical signal can be separated into pulsatile and non-pulsatile components (by assessing AC vs DC current) & an R value is calculated (AC660nm/DC660nm divided by AC940nm/DC940nm).
Output:
- Numerical display
- Graphical display - Plethysmograph
Potential errors:
- Movement artefact - pulsox are vulnerable to motion (increased ambient light input, etc)
- Optical shunting - if the probe is wrong size, or the light passes through the length of an artery travelling horizontally
- Too much ambient light
- Electromagnetic interference - may get creation of microcurrents in the equipment
- Poor peripheral perfusion
- Calibration - low sats unlikely to be accurate
- Coloured dye and nail polish may affect the absorption
- Abnormal haemoglobins - carboxyhaemoglobin can be detected as oxyhaemoglobin, showing falsely high sats. Methaemoglobin tends to show sats towards 85%
Describe the principles of capnography, including calibration, sources of errors and limitations
(syllabus)
End tidal CO2 = CO2 in expired gas measured at the mouth (or ETT) at the end of expiration
- There is usually a difference of ~2-5mmHg.
- This explained by alveolar dead space, which is minimal in healthy adults
- Dead space describes areas of the lung that are ventilated but not perfused. Gas in dead space is similar in composition to inspired air, so mixing of this with air from ventilated lung units results in the dilution of CO2 content of expired air. Increasing alveolar dead space will cause in increase in the PaCO2-EtCO2 difference
Causes for an increased PaCO2-EtCO2 difference:
- Patient factors:
- Decreased regional pulmonary perfusion
- PE/fat embolism/air embolism
- Decreased global pulmonary perfusion
- Profound hypovolaemia - haemorrhagic shock
- Cardiac arrest
- Cardiac failure (RHF)
- Changes in ventilation
- High PEEP/IPPV - increase in Wests Zone 1
- High FiO2 for extended periods - decrease in PVR & redirection of pulmonary flow towards poorly ventilating alveoli
- Large shunt fraction (>30%)
- Decreased regional pulmonary perfusion
- Equipment/measurement factors (may cause either increase or decrease in PaCO2-EtCO2 difference)
- Loss of calibration of sampling unit
- Leaks, occlusion of sampling line
- Excess H2O in water trap interfering with measurement
- Interference from other gases (N2O) mistaken for CO2
- Timing of CO2 measurement - needs to be at end expiration
Describe the methods of measurement of oxygen and carbon dioxide tension in blood
Methods of measurement of oxygen:
The platinum oxygen cathode:
- Consists of a platinum cathode and Silver anode bathed in KCl solution. Both anode and cathode are placed in an aqueous solution, which contains dissolved oxygen (pt blood sample) and connected by a battery which generates a potential difference (usually 600-700mV)
- The oxygen is reduced at the platinum electrode (O2 + 4H+ + 4e- = 2H2O) and silver is oxidise at the silver anode (4Ag –> 4Ag+ + 4e-; 4Ag+ + 4Cl- –> 4AgCl) - AgCl forms a crust around the silver electrode
- The flow of electrons through the circuit can be detected by a galvanometer
- A polarogram (current vs voltage graph) can be plotted for pO2
- The shape of this graph is sigmoid - at low voltages, the flow of current is limited by amount of available energy. At high voltages, current reaches a plateau as the flow of current become limited by oxygen available to be reduced at the cathode (limited by diffusion of oxygen to cathode). The higher the pO2, the faster the diffusion and higher the voltage required to reach the plateau
- ABG machines vary the voltage in order to find the plateau and infer the pO2 from the required voltage
- Therefore, the oxygen cathode measure the electrical activity equivalent of oxygen tension
The Clark electrode:
- Uses the same principle of the platinum cathode, but consist of the silver anode and platinum cathode both bathed in a Cl rich electrolyte solution with an oxygen permeable membrane (porous tetrafluroethylene) separating the aqueous solution (blood gas sample) from the electrolyte with electrodes.
- The rate of response of the electrode depends on membrane thickness, and diffusion distance post membrane (so electrodes are pushed right up against membrane)
Methods of measurement of carbon dioxide:
The Servinghaus electrode:
- The pH is calculated by measuring the generated potential difference using the Nernst equation (at 37 degrees, the potential difference changes by -61.5mV per pH unit
How can work of breathing be minimised?
Minimise elastic forces
* Ensure tidal volume breathing over favourable portion of the compliance curve – use CPAP/PEEP if needed.
* Avoid very large tidal volumes.
* Ensure adequate surface tension (Only relevant in infant respiratory distress syndrome)
Minimise non-elastic forces
* Minimise airway resistance
* o Avoid high resp rate (increased flow rate = increased airway resistance in laminar flow but also increases risk of turbulent flow with even higher resistance), consider cautious PEEP in obstructive airway disease (lowers resistance at cost of increased risk of gas trapping)
* o Bronchodilators/steroids etc
* o Heliox
Minimise viscous tissue resistance
* o Treat APO/pneumonia/sputum plugs
* o Escharotomy for burns
* o Empty abdominal contents (5 F’s), harder to correct foetus or fat.
* o Decompress pleural gas or fluid.
* o Sit patient up
Treat metabolic acidosis
Describe the physiology of the pulmonary circulation
Volume:
- Contains ~500mL of blood (10% in capillaries, 45% in each arteries and veins)
- Can halve or double to adjust for posture, respiratory effort, and changes in systemic circulation
Flow:
- Receives all of cardiac output (6L/min)
- Regional distribution of perfusion:
– Gravity and posture have a large effect on the distribution of flow.
– Dependent parts of the lung are perfused to a greater degree
– The interplay between pulmonary arterial pressure, pulmonary venous pressure, alveolar pressure and lung interstitial pressure can be described by West’s zones
Pressure:
- Low pressure system (25/8mmHg vs systemic - MAP 90mmHg)
- This is just enough pressure to perfuse apices
- Decrease in pressure (eg. shock states) –> decreased perfusion to apices + create West zone 1
Resistance:
- normal vascular resistance = 100dynes.s.cm (-5)
How do time constants affect the respiratory system?
Physiological:
* Compliance
— Observed difference between static and dynamic compliane - static compliance measured when all fast and slow alveoli have equilibrated
— Compliance in apical lung units is lower (as intrapleural pressure difference is lower). Therefore time constants are faster - ie. these alveoli fill up quickly
* V/Q matching - at high RR, slow alveoli don’t have time to fill and ventilation preferentially diverts to fast alveoli (V/Q mismatch for slow alveoli)
In disease:
* Asthma/COPD - increased airway resistance = increased time constant
* Emphysema - increased compliance = increased time constant
* Pulmonary fibrosis - decreased compliance = faster time constant


