Cardiac physiology Flashcards
Describe the structure and functional significance of the excitatory, conductive and contractile elements of the heart
Syllabus

Compare the structure, function and coronary circulation of the right and left ventricle
Exam

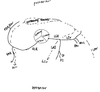
Describe the coronary circulation
own
LCA - left coronary artery
LAD (to diagonal 1&2): RV, LV, AV bundle, anterior 2/3 of IVS
LCx (to OM1&2): LA, LV
RCA- right coronary artery: RA, RV, SA+AV node, usually posterior 1/3 of IVS (80-85%)
Coronary arterial dominance: RCA, LCx or both gives rise to the posterior descending artery (PDA), which supplies the myocardium of the inferior 1/3 of the IVS
Return:
- coronary sinus returns in between IVC and TV with saturation <30%
- SCV = small cardiac vein; GCV = Great cardiac vein, LMV = left marginal vein
- thebesian vein (not shown) - directly into all the four chambers
Describe the normal pressure and flow patterns (including velocity profiles) of the cardiac cycle
Syllabus
Events during systole:
- Isovolumetric ventricular contraction
- Corresponds with peak of R wave & Phase 0 (rapid Na+ influx) of ventricular myocyte AP
- Ventricles begin to contract, increasing ventricular chamber pressure
- MV + TV close
- Fixed ventricular volume
- Early ejection
- Contracting ventricles achieve a pressure high enough to open the aortic and pulmonic valves, and rapidly empty into the systemic and pulmonary circulations
- This period corresponds to Phase 2 (plateau, rapid calcium influx) of the cardiac myocyte action potential
- On the surface ECG, the end of this phase corresponds to the beginning of the T wave
- Late ejection
- Begins when ventricular pressure starts to drop, & ends with closure of AV + PV
- The end of this period corresponds to the peak of the T wave on the surface ECG
- Corresponds to Phase 3 (repolarisation) of the cardiac myocyte action potential
Events during diastole:
- Isovolumetric relaxation
- Ventricles relax without any change in volume & pressure drops until TV + MV open
- Corresponds to end of the T wave on ECG & the end of Phase 3 of the action potential
- Early rapid diastolic filling
- Ventricles have pressure lower than atrial pressure –> fill rapidly
- 80% of the ventricular end-diastolic volume is achieved during this phase
- Coronary blood flow is maximal during this phase
- Late slow diastolic filling
- Ventricular & atrial pressures equilibrate and the atria act as passive conduits for ventricular filling
- The end of this phase corresponds to end of the P-wave ECG
- Atrial systole
- The atria contract (right first, then left)
- Increases pressure in the ventricles up to the end-diastolic pressure, and adds about 20ml of extra volume to the end-diastolic volume
- Starts at end of the P-wave on ECG, & finishes during the PR interval
- End of this phase corresponds to the peak of the R wave, or the Phase 0 (rapid sodium influx) of the ventricular myocyte action potential

Describe the fetal circulation & the circulatory and respiratory changes that occur at birth
Syllabus
Fetal circulation
Oxygenated blood:
- Blood is oxygenated in placenta, and travels to fetus via umbilical vein. This connects to the IVC via ductus venousus
- Blood flows from fetal IVC to RA. From there
- Most blood travels to LA via foramen ovale (L–>R shunt)
- Some travels to RV, then to pulmonary trunk
- Blood in pulmonary trunk travels to aorta via ductus arteriosus
- From LA, blood flows from LV –> aorta –> tissues
Deoxygenated blood:
- Travels from fetal tissues to heart via IVC
- Some blood travels via umblicial arteries to the placenta to become oxygenated
At birth
- Lungs are aerated with the first breaths (purges liquid, creates FRC). This decreases PVR
- SVR increased by clamping umbilical cord
- RV output channelled into pulmonary circulation rather than systemic
- Increased LA pressure (increased pulmonary blood flow + SVR) –> reverses flow across foramen ovale (closes immediately)
- Increased aortic pressure reverses flow across ductus arteriosus (functionally closes over ~24hrs, anatomically over several days)
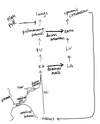
Explain the ionic basis of spontaneous electrical activity of cardiac muscle cells
Syllabus
Electrical activity in the heart is driven by the influx and efflux of ions across myocardial cell membranes according to chemical concentration differences and difference in electrical charges of ions intracellularly vs extracellularly
- In the ventricles at rest, the electrical potential difference (voltage - Vm) (or Resting membrane potential (RMP)) is -90mV (intracellularly negative)
- The RMP in -90mV (threshold potential = -70mV) in all fast-response action potentials (atrial + ventricular myocytes)
- RMP is -60mV (threshold potential = -40mV) in slow-response action potentials (in pacemaker cells in SA node)
- Damage to myocytes can make RMP less negative, eg ~-50mV, which can be a cause for spontaneous activity of myocytes
- The chemical concentration gradient at rest is similar between all myocardial cells:
- Intracellular K = 135mmol/L –> 4mmol/L (extracellular)
- Na = 10mmol/L –> 145mmol/L
- Ca = 1x10-4 mmol/L –> 2mmol/L
- The permeability of myocardial cells to these ions is dependent on the expression of ion-specific channels along the cell membrane, and the activation of these channels, which varies between different type of myocardial cells. Some of these include:
- Voltage-gated Na+ channels - important in AP of atrial + ventricular cells
- Ca++ channels - important particular in pacemaker cells
- Na+/K+ ATPase - active pump, important in all cells
- Potassium specific channels - myocytes are particularly permeable to K+ due to a large number of K+ specific channels
- Note: phase 2 is less pronounced in atrial vs ventricular APs
Describe the normal processes of cardiac excitation and electrical activity
Syllabus
The action potential of a ventricular myocyte:
- Phase 0 = rapid depolarisation of membrane. Vm -90mV –> +50mV
- Fast Na+ channels (activated - m gate opens) open for a small amount of time - Na+ rapidly moves intracellularly due to large concentration difference (sodium current (iNa))
- There is a slow initial increase in Vm from -90mV –> -70mV (this is the threshold potential)
- Phase 1 = partial/rapid repolarisation.
- Rapid decrease in Na+ permeability as voltage-gated Na channel closes (inactivated - h gate closes)
- Voltage-gated K+ channels open, leading to K+ efflux (down both electrical and concentration gradient)
- Phase 2 = plateau
- 150-200ms
- Ca2+ influx balances out ongoing K+ efflux (Ito = transient outward K+ current) . Ca2+ influx triggers Ca2+ release from sarcoplasmic reticulum
- L-type Ca2+ channels open - activated initial when Vm = -30mV. Once activated, they are slow to close and they are blocked by calcium antagonists
- Phase 3 = rapid repolarisation
- Massive K+ efflux due to opening of voltage-gated slow K+ channels and closure of voltage gated Ca2+ channels
- iK1 channels (aka Kir) - inwardly rectifying. This means they more easily allow K influx than efflux (which saves the cell from massive K efflux during phase 2)
- Contributes more substantially to the later repolarisation phase - conductance increases as Vm becomes more negative
- iK channels are delayed rectifier channels. Activated at voltages towards end of phase 0 but are very slow to open
- IKr refers to currents that activate more rapidly
- IKs refers to currents that activate more slowly
- iK1 channels (aka Kir) - inwardly rectifying. This means they more easily allow K influx than efflux (which saves the cell from massive K efflux during phase 2)
- Massive K+ efflux due to opening of voltage-gated slow K+ channels and closure of voltage gated Ca2+ channels
- Phase 4 = resting potential
- High K+ permeability through K+ channels (maintained by the inward rectifying IK1 potassium current)
- Plateau
Action potential for pacemaker cells:
- Phase 4 - max membrane potential -65mV, with no real resting potential - slow depolarisation secondary to If (funny current) - mixed sodium-potassium current
- Activated when Vm
- Activated by cAMP, which is increased by sympathetic stimulation
- (Target of ivabradine)
- When Vm = -40mV, T-type Ca2+ channels open
- Phase 0 - Rapid depolarisation when L-type Ca2+ channels open - Rapid Ca2+ influx (however this is slower than myocyte depolarisation)
- Vm peak = 20mV
- Phase 3 - repolarisation via increased K+ permeability (K+ efflux). Mediated by Ikr, Iks and IK1 currents

Describe the abnormal processes of cardiac excitation and electrical activity
Syllabus
- Abnormal cardiac excitation can be classified as:
- Automatic: where the cells act as a pacemaker
- Triggered: where the cells depolarise unexpectedly in response to an external stimulus (early and late afterdepolarisations)
- Abnormal automaticity is the spontaneous generation of action potentials in excitable cardiac tissues which are usually not expected to act as pacemakers (eg. Purkinje cells). Abnormal automaticity can be caused by:
- Sustained direct current (catelectrotonus)
- Hypokalemia
- Mechanical stretch
- Barium toxicity
- Early afterdepolarisations are triggered depolarisations which occur during Phase 3, and which are promoted by anything which prolongs the repolarisation:
- Hypokalemia
- Antiarrhythmics and other drugs which prolong the QT interval
- Hypoxia
- Acidosis
- Catecholamines
- Late afterdepolarisations are triggered depolarisations which occur during Phase 4, and which are promoted by anything that might increase the intracellular calcium:
- Hypercalcemia
- Tachycardia
- Catecholamines
- Digitalis glycosides
- Anything that increases intracellular sodium, eg. sodium channel blockers
Explain the Frank-Starling mechanism and its relationship to excitation-contraction coupling
Syllabus
Frank-Starling mechanism
- Stroke volume increases with increasing preload
- The relationship is non-linear and begins to plateau at high preload/end-diastolic volumes
- Increased contractility will increase stroke volume at every EDV. Decrease contractility will decrease SV at every EDV, and may result in decreased SV with increasing EDV
Excitation-contraction coupling
- Describes the process of depolarisation of myocytes causing Ca2+ mediated Ca2+ release from the sarcoplasmic reticulum, which causes sarcomere contraction
- How this relates to Frank-Starling mechanism:
- With increasing sarcomere length, sarcomeres become more sensitive to calcium somehow and the force of their contraction increases as a result
- Current theory of why this happens is that by stretching a cylindrical myocyte without changing its volume, the fibre becomes thinner. Thinning of the myocyte brings thick and thin filaments closer together, which should increase the likelihood of the force-generating crossbridge reactions from taking place

Define the components and determinants of cardiac output including the effects of positive pressure ventilation (Syllabus)
Cardiac output definition: the volume of blood ejected by the heart per unit time (L/min)
CO = HR x SV
Determinants of cardiac output:
- Heart rate - this is under the influence of autonomic control, and can be targeted by many drugs
- Increasing heart rate does not necessarily cause a proportionate increase in CO, as increasing heart rate can cause a decrease in stroke volume (due to shortening of diastolic filling time)
- Stroke volume - the volume of blood pumped out of the LV during each systolic cardiac contraction
- Preload - can be defined in relation to pressure or volume. In relation to pressure, it is the tension on the myocardial sarcomere just prior to contraction (end-diastolic pressure). In relation to volume, it is the myocardial sarcomere length just prior to contraction (end-diastolic volume). Increased preload leads to an increase in stroke volume. However there are a number of factors that affect preload:
- Intrathoracic pressure
- Ventricular compliance
- Effectiveness of atrial kick
- Total venous blood volume (and venous vascular compliance)
- Duration of diastole
- End-systolic volume
- Afterload - the resistance to ventricular ejection. Consists of:
- Myocardial wall stress
- Ventricular wall thickness
- Ventricular transmural pressure - dependent on intrathoracic pressure, ventricular output impedance
- Input impedence
- Arterial resistance (which has many influencing factors - eg. Arterial compliance, inertia of blood column)
- Myocardial wall stress
- Contractility
- Increased contractility improves stroke volume at any given preload or afterload. It is affected by:
- Heart rate (Bowditch effect)
- Afterload (Anrep effect)
- Preload (Frank-Starling mechanism)
- Intra- and extra-cellular calcium concentration
- Temperature
- Increased contractility improves stroke volume at any given preload or afterload. It is affected by:
- Preload - can be defined in relation to pressure or volume. In relation to pressure, it is the tension on the myocardial sarcomere just prior to contraction (end-diastolic pressure). In relation to volume, it is the myocardial sarcomere length just prior to contraction (end-diastolic volume). Increased preload leads to an increase in stroke volume. However there are a number of factors that affect preload:
The effects of positive pressure ventilation:
- PPV decreases afterload by decreasing the ventricular transmural pressure, which is an important component of myocardial wall stress (Law of Laplace)
Where P = transmural pressure & a decrease in transmural pressure will result in decrease in wall stress/afterload
Define the components and determinants of cardiac output including the effects of positive pressure ventilation (Syllabus)
Cardiac output definition: the volume of blood ejected by the heart per unit time (L/min)
CO = HR x SV
Determinants of cardiac output:
- Heart rate - this is under the influence of autonomic control, and can be targeted by many drugs
- Increasing heart rate does not necessarily cause a proportionate increase in CO, as increasing heart rate can cause a decrease in stroke volume (due to shortening of diastolic filling time)
- Stroke volume - the volume of blood pumped out of the LV during each systolic cardiac contraction
- Preload - can be defined in relation to pressure or volume. In relation to pressure, it is the tension on the myocardial sarcomere just prior to contraction (end-diastolic pressure). In relation to volume, it is the myocardial sarcomere length just prior to contraction (end-diastolic volume). Increased preload leads to an increase in stroke volume. However there are a number of factors that affect preload:
- Intrathoracic pressure
- Ventricular compliance
- Effectiveness of atrial kick
- Total venous blood volume (and venous vascular compliance)
- Duration of diastole
- End-systolic volume
- Afterload - the resistance to ventricular ejection. Consists of:
- Myocardial wall stress
- Ventricular wall thickness
- Ventricular transmural pressure - dependent on intrathoracic pressure, ventricular output impedance
- Input impedance
- Arterial resistance (which has many influencing factors - eg. Arterial compliance, inertia of blood column)
- Myocardial wall stress
- Contractility - the change in peak isometric force at a given initial fibre length and given afterload + heart rate
- Increased contractility improves stroke volume at any given preload or afterload. It is affected by:
- Heart rate (Bowditch effect)
- Afterload (Anrep effect)
- Preload (Frank-Starling mechanism)
- Intra- and extra-cellular calcium concentration
- Temperature
- Increased contractility improves stroke volume at any given preload or afterload. It is affected by:
- Preload - can be defined in relation to pressure or volume. In relation to pressure, it is the tension on the myocardial sarcomere just prior to contraction (end-diastolic pressure). In relation to volume, it is the myocardial sarcomere length just prior to contraction (end-diastolic volume). Increased preload leads to an increase in stroke volume. However there are a number of factors that affect preload:
The effects of positive pressure ventilation:
- PPV decreases afterload by decreasing the ventricular transmural pressure, which is an important component of myocardial wall stress (Law of Laplace)
Where P = transmural pressure & a decrease in transmural pressure will result in decrease in wall stress/afterload
Define preload and its components
own
PRELOAD and its determinants:
- Pressure with which the ventricle fills
- Intrathoracic pressure (which will affect RV & LV differently)
- Contribution of ‘atrial kick’, which in turn depends on atrial contractility + synchrony (eg SR vs AF), valvular function, LV end systolic volume & LV compliance. This is generally a small contribution of volume (~20% of LVEDV). Specific conditions in which the atrial kick can become more significant:
- Tachycardia
- Diastolic heart failure (HFpEF)
- Aortic stenosis
- Mitral stenosis
- RA pressure (surrogate: CVP) - rate of blood flow back to the heart is determined by the pressure gradient between MSFP and RAP. CVP used to be used as a marker of preload, but it is affected by multiple factors
- MSFP, which in turn depends on total venous blood volume and venous vascular compliance
- MSFP = pressure in the systemic circuit only, and is the pressure that the vascular walls exert on its fluid content, in the absence of flow
- The mean circulatory filling pressure (MCFP) is MSFP plus heart and pulmonary vasculature
- ~15% of total blood volume is contributing to MSFP - 85% is ‘unstressed’
- Compliance of the ventricle
- Pericardial compliance
- Ventricular wall compliance, which depends on: diastole (eg. Duration) and myocardial structure (eg. wall thickness, amount of fibrous tissue)
- Lusitropy (relaxation) of muscle
- End-systolic volume of the ventricle (dependent on afterload)
Define afterload and its components
own
AFTERLOAD and its determinants:
- Myocardial wall stress - described by Law of Laplace. The law is described as wall stress ‘proportional to’ Pr/h, because ventricles aren’t spherical, and the shape changes with variation in the cardiac and respiratory cycle
- Radius of ventricle - (ie- EDV, ie preload) - increased diameter increases wall stress
- Ventricular transmural pressure - (ventricular cavity pressure minus intrathoracic pressure)
- Thickness of ventricular wall - increased thickness decreases wall stress (stress distributed among larger number of working sarcomeres)
- Input impedance
- Ventricular outflow tract resistance
- Arterial compliance - aortic + peripheral
- Decreased proximal aortic compliance will increase wall stress and decrease flow (60% of every stroke volume is stored in distended arterial capacitance vessels (have a tunica media which allows them to expand))
- Peripheral compliance:
- Normally, pulse pressure wave propagates to distal circulation at about 1m/s, which is reflected from arterioles and returns to the heart during diastole - helps fill coronaries
- Decreased compliance of peripheral circulation increases the speed of pulse wave propagation & the wave returns sooner, in systole which adds extra pressure to the aortic pressure
- Inertia of blood column
- Arterial resistance (can be described by Poiseuille equation), where vessel radius is most important variable (raised to power of 4)
Define contractility and its determinants, including description of the cardiac reflexes
own
CONTRACTILITY and its determinants:
- Surrogates for contractility
- ESPVR - used to describe the maximal end-systolic pressure which can be achieved with increasing end-diastolic volume. The slope of the ESPVR line describes contractility - steeper slope = more contractile ventricle. Pitfalls: the ESPVR slope decreases progressively as ventricular size increase without this change necessarily indicating a change in contractility
- dP/dT - maximum rate of change in left ventricular pressure during isovolumetric contraction. Decreased slope = decreased contractility. Preload dependent and (relatively) afterload independent. Pitfalls: raised diastolic BP will result in rise of dP/dT peak; dP/dT is dependent on heart rate (hard to assess effect of an ionotrope if also chronotrope properties
- CVS parameters
- Preload - increased preload will cause an increase in sarcomere stretch and increase contractility according to Frank-Starling relationship
- Afterload - Anrep effect –> an increased in afterload will cause an increase in contractility (Frank-Starling mechanism - increased afterload –> increased end systolic volume –> sarcomere stretch –> increased contractility. This is followed by a gradual increase in intracellular calcium (aldosterone mediated increase in intracellular Na+, which then increases activity of Na+/Ca+ exchanger))
- Heart rate - Bowditch effect - increase in heart rate will increase contractility (mechanism: relaxation is driven by expulsion of intracellular calcium into sarcolemma or extracellular space. With decreased time for expulsion (increased heart rate), calcium builds up intracellularly, and produces increase in contractility). Note: this only becomes relevant at very high heart rates (~200), at which point this effect would be offset by a decrease in SV due to decreased time for diastolic filling.
- Woodworth effect - an increase in contractility after a prolonged period between contractions
- Biochemical/cellular factors
- Calcium concentration - central to excitation-contraction coupling. Factors influencing intracellular calcium concentration:
- Catecholamines - increased activity of β1-receptors –> increases cAMP –> increased PKA activity –> increased intracellular calcium
- Ischaemia - impairment of contractile function thought to be due to a decrease in the ability of intracellular calcium to trigger the release of more calcium from the sarcolemma
- Extracellular calcium - contractility decreases linearly with decreasing extracellular calcium concentrations
- Temperature
- Moderate hypothermia decreases contractility (catecholamines lose affinity for receptors, decreased cardiac myofilament sensitivity to calcium & activity of cardiac actin-activated myosin ATPase decreases
- Calcium concentration - central to excitation-contraction coupling. Factors influencing intracellular calcium concentration:
Describe the effects of positive pressure ventilation on the cardiovascular system
Part-syllabus
The effects of positive pressure ventilation:
- PPV decreases afterload by decreasing the ventricular transmural pressure, which is an important component of myocardial wall stress (Law of Laplace)
Where P = transmural pressure & a decrease in transmural pressure will result in decrease in wall stress/afterload
(From F10i) - PPV effects on the cardiovascular system
- RV + pulmonary circulation:
- Increase intrathoracic pressure is transmitted to central veins + RA –> decreased RV preload
- Note: only about 25% of PEEP is transmitted to central veins
- PPV increases PVR –> Increases RV afterload
- This has the net effect of decreasing RV stroke volume
- Increase intrathoracic pressure is transmitted to central veins + RA –> decreased RV preload
- LV + systemic circulation
- PEEP decreases LV compliance (increased RV afterload –> dilatation of RV –> bulging of IV septum into LV during distole (LVEDP < RVEDP))
- Decreases preload (decreased pulmonary venous pressure)
- Decreases afterload - (decrease in LV end-systolic transmural pressure + increased gradient between intrathoracic aorta and extrathoracic systemic circuit - ie. Pressure gradient favours blood exiting the thorax in PPV vs spont vent where neg IT pressure creates a force that opposes flow out of thorax)
- Overall, increased PEEP causes decreased CO + decreased myocardial O2 demand
Describe myocardial oxygen supply and the conditions that may alter it
Part syllabus
SUPPLY - coronary circulation
Anatomy
- Origin: sinus of Valsalva
- Artery and Supply:
- LCA
- LAD (to diagonal 1&2): RV, LV, AV bundle, anterior 2/3 of IVS
- LCx (to obtuse marginal 1&2): LA, LV
- RCA: RA, RV, SA+AV node, usually posterior 1/3 of IVS (80-85%)
- Coronary arterial dominance: RCA, LCx or both gives rise to the posterior descending artery (PDA), which supplies the myocardium of the inferior 1/3 of the IVS
- LCA
- Return:
- Coronary sinus
- return in between IVC and TV
- saturation <30%
- thebesian veins
- directly into all the four chambers
- Coronary sinus
Physiology
- Normal coronary blood flow = 5% of cardiac output (80mL/min/100g)
- Perfusion pressure = aortic diastolic pressure - max [LVDP or RAP]
- LV is perfused during diastole due to high LV pressure during systole
- RV is perfused during systole
- Coronary O2 delivery:
- DO2 = CBF x CaO2 (arterial O2 content)
Factors affecting coronary flow:
- Metabolic demand dominates control of the coronary artery flow
- oxygen ER in coronary circulation is very high (60-70%)
- Coronary arterial blood flow increases to match myocardial oxygen demand, instead of increasing O2-ER. Exercise can increase CBF by 4-5 fold
- Main mechanism: Vasodilation by metabolic substrates
- adenosine, NO, prostaglandins, CO2, lactate, H+
- ATP-sensitive K+ channel opens in response to decreased ATP -> membrane hyperpolarisation and vasodilation
- Other mechanisms:
- myogenic autoregulation
- sympathetic nervous system: directly constricts coronary artery, but enhances myocardial metabolism demand by tachycardia; thereby indirectly causing vasodilation
Describe myocardial O2 demand and the conditions that may alter it
Part syllabus
DEMAND + CONSUMPTION
- Quiescent myocytes are metabolically active - with oxygen consumption of 2.24mL/100g/min (eg in cardiac arrest). This increases to 6-8mL/100g/min in contracting myocytes in resting state, to 90ml/100g/min at maximum ionotropy
- Temperature affects basal metabolic rate - hypothermia decreases basal metabolic rate & decreases O2 consumption by decreasing HR & contractility
- Metabolic fuels
- FFAs (65%), Glucose (15%), Lactate and pyruvate (12%), Amino acids (3%), anaerobic glycolysis (5%)
- Multiple factors changes these ratios:
- Availability of substrate
- Exercise - heart increases lactate intake
- In hypoxaemic environments, glucose + FFA metabolism is turned off (as they require O2) & anaerobic glycolysis occurs - heart becomes net producer of lactate rather than net consumer
- ATP is rapidly depleted in absence of substrate supply
- Different physiological and pathological states
- Adult heart uses fatty acids, foetal heart uses glucose and lactate
- Ischaemia, hypertrophy and increased afterload can increase myocardial glucose use
- Availability of substrate
- Myocardial oxygen demand can be broken up into external & internal work, as shown below

Compare and contrast the supply and demand of oxygen for the right and left ventricle
Past question (2016, Paper 2, Q7)

Describe and explain cardiac output curves. Outline changes to cardiac output with enhanced and reduced cardiac performance on a diagram
own
- Similar to the Frank-Starling curve, but x-axis is RAP (rather than EDP) and y-axis is CO (rather than SV)
- Important features of this curve:
- It is linear at low RAPs (the range of pressures and preloads for which the length-tension relationship is optimised. Slope represents different states of contractility)
- Plateau - CO stops increasing beyond a certain RAP (~4mmHg)
- X-intercept - where curve hits x-axis
- The curve shifts upwards with enhanced cardiac performance (increased ionotropy, increased heart rate, reduced afterload)

Describe vascular function curves & their interaction with cardiac output curves
own
The vascular function curve:
- Describes what happens to venous return with changes in RAP
- Important features of this graph:
- MSFP - when MSFP = RAP, venous return will be 0
- X-intercept- MSFP represents a limit of what RAP can be
- Plateau - when RAP is 0 or negative, this doesn’t apply any ‘suction’ to the central veins - they tend to collapse (ie. Decreasing RAP to below atm does nothing to increase CO
Intersection of the two curves:
“Guyton diagram”
- Steady state operating point - where CO = venous return (defining characteristic of CVS)
- With an increase in volume:
- Venous return curve shifts to right
- Steady state operating point moves up CO curve
- MSFP shifts to right (due to increased volume)
- An identical effect would be seen if venous compliance was changed (eg. With a vasopressor) - increased smooth muscle tone would increase the size of the ‘stressed volume’ –> increases MSFP
- With an increase in contractility:
- The cardiac output curve shifts upwards (with same x-intercept)
- Venous return increases & RAP decreases
- With an increase in peripheral vascular resistance (ie arteriolar smooth muscle tone):
- Venous blood volume would decrease (increase resistance to flow out of arterial system)
- MSFP remains the same (total blood volume unchanged)
- Steady state point would shift down (downward shift of curve with MSFP constant) - although note, this would be associated with decreased RAP according to the figure, which represents one of the limitations of the Guytonian model

Describe the pressure-volume relationships of the ventricles and their clinical applications
Syllabus
- Plot of LV volume vs pressure
Information that can be obtained from a PV loop:
- Volumes:
- End diastolic volume (EDV) - when mitral valve closes
- End systolic volume (ESV) - when aortic valve closes
- SV (stroke volume) - EDV minus ESV
- Ejection fraction - ratio of stroke volume to end diastolic volume
- Pressures:
- Diastolic pressure - when aortic valve opens
- Systolic pressure - the peak of the curve
- End-systolic pressure - when aortic valve closes
- Pressure/volume relationships:
- Contractility - the slope of the ESPVR is a surrogate measure for contractility - steeper = more contractile
- Ventricular elastance: the EDPVR line is a measure of ventricular elastance (change in pressure per unit change in volume) not compliance (change in volume per unit change in pressure)
- Increased elastance (increased resistance to filling), such as in diastolic dysfunction, will be seen as an increased slope in the EDPVR line
- Afterload
- Usually given by LaPlace law (afterload = Pr/h)
- Surrogate is Arterial elastance, Ea, which is given by the slope of the line connecting the end-systolic pressure to the x-axis at the point of maximal preload (mitral valve closure)
- This slope is calculated by end systolic pressure divided by stroke volume
- Areas:
- External work (stroke work) - given by the area contained within the PV loop
- Internal work (potential energy stored within LV wall) - area under ESPVR line

Outline the physiological factors that influence cerebral blood flow.
Past question
Cerebral circulation: - Cerebral blood flow is supplied by the carotid (70%) and vertebral (30% arteries) ○ It is usually 50ml/100g/min, or 14% of normal cardiac output - It is described by the Ohm equation, Q = (Pa- Pv) / R, where ○ (Pa- Pv) is the cerebral perfusion pressure (CPP) ○ R is the cerebral vascular resistance - Cerebral perfusion pressure = MAP - (ICP or CVP, whichever is higher) ○ The higher the ICP (or CVP), the lower the CPP, if the MAP remains stable - Cerebral resistance (R) = (8 l η) / πr4, where ○ l = length of the vessel ○ η = viscosity of the blood ○ r = radius of the cerebral vessels, which is the main variable susceptible to regulation - Cerebral autoregulation is a homeostatic process that regulates and maintains cerebral blood flow (CBF) constant and matched to cerebral metabolic demand across a range of blood pressures (MAP: 50-150 mmHg). This is mediated by the myogenic mechanism, local mediators (nitric oxide). It is affected by: ○ PaCO2: increased PaCO2 leads to increased CBF § Change in periarteriolar pH --> change in NOS activity --> catalyses cGMP production --> decreased intracellular [Ca2+] --> vasodilation (decreased cerebral vascular resistance) § Increase in CBF is 1-2mL/100g/min for every 1mmHg increase in CO2 ○ PaO2: PaO2 falling below 50 mmHg leads to exponentially increased CBF
Outline determinants of preload
Vascular factors
* MSFP (venous blood volume + venous compliance)
Factors impeding venous return
* High intrathoracic pressure
* High intra-abdominal pressure (including caval compression syndrome in pregnancy)
* RAP (Guyton’s curves)
Myocardial factors
* Contribution of atrial kick (impaired in AF, arrhythmias)
* Valvular competence (mitral stenosis)
* Ventricular compliance
— pericardial compliance
— ventricular wall compliance
— end systolic volume (depends on afterload)
Outline determinants of afterload
- Myocardial wall stress -(Laplace) - Pr/h
○ Radius
○ Ventricular transmural pressure - (cavity pressure minus ITP)
○ Thickness of wall - increased thickness decreases wall stress ( - Input impedance
○ Ventricular outflow tract resistance
○ Arterial compliance - aortic + peripheral
— Decreased proximal aortic compliance will increase wall stress and decrease flow (60% of stroke volume stored in arterial capacitance vessels (tunica media allows expansion))
— Peripheral compliance:
□ Pulse pressure wave–> distal circulation at 1m/s–> reflected from arterioles –>returns to heart during diastole (helps fill coronaries)
□ Decreased compliance –>increases the speed of pulse wave propagation –> wave returns sooner, in systole –> adds pressure to aortic pressure - Inertia of blood column
- Arterial resistance (can be described by Poiseuille equation), where vessel radius is most important variable (raised to power of 4)


