Exam II Flashcards
what stabilizes the structures in the secondary structures
hydrogen bonds
what is the configuration of side chains in alpha helix?
facing away from the center
what configuration do most peptide chains take (cis or trans)?
trans, exception is proline, which sometimes forms cis (10%) d/t less steric hindrance.
how is the backbone of a polypeptide chain described
by torsion angles, aka dihedral angles
the backbone of a polypeptide chain is described as rigid? What causes this?
The peptide group has a rigid, planar structure because of resonance interactions that give the peptide bond 40% double bond character
Both the α helix and the β sheet are called regular secondary structures because ?
they are composed of sequences of residues with repeating φ and ψ values.
T/F: the alpha helix is always right handed
true because it’s the only conformation of helix that is favorable according to the ramachandran diagram
where are the R groups pointing in alpha helix?
outward and downward
what is the central difference between beta sheets and alpha helices?
in beta sheets hydrogen bonding is between neighboring polypeptide chains rather than within one polypeptide
where are the side chains pointing in beta sheets?
perpendicular to the sheet and alternating sides, such that 2 R groups will be on the same side every 7 angstroms

what accounts for the pleated appearance of the beta sheet?
the conformations of deal hydrogen bonding make the structure deviate from the phi and psi angles of 180° of its fully extended form
Parallel β sheets containing fewer than five strands are rare. Why is that?
This observation suggests that parallel β sheets are less stable than antiparallel β sheets, possibly because the hydrogen bonds of parallel sheets are distorted compared to those of the antiparallel sheets
The geometry of a particular β sheet is said to be a compromise between
optimizing the conformational energies of its polypeptide chains in terms of R group interactions and preserving its hydrogen bonding.
because the interactions between chiral R groups causes a twist in the sheet that distorts and weakens the β sheet’s interchain hydrogen bonds.
T/F: the link between tandem parallel strands must be a crossover connection that is out of the plane of the β sheet
true

what is a β bend
- Polypeptide segments with regular secondary structure such as α helices or the strands of β sheets are often joined by stretches of polypeptide that abruptly change direction.
- Such reverse turns or β bends (so named because they often connect successive strands of antiparallel β sheets) almost always occur at protein surfaces.
- They usually involve four successive amino acid residues arranged in one of two ways, Type I and Type II, that differ by a 180° flip of the peptide unit linking residues 2 and 3
- In Type II turns, the oxygen atom of residue 2 crowds the Cβ atom of residue 3, which is therefore usually Gly.
- Residue 2 of either type of turn is often Pro since it can assume the required conformation.

Keratins have been classified as either α keratins, which occur in 1 , or β keratins, which occur in 2
1 mammals
2 birds and reptiles
What 2 factors are principally responsible for the structure of a coiled coil?
- Its primary structure: the central segment of the polypeptide units in a coiled coil each have a 7-residue pseudorepeat of a-b-c-d-e-f-g, where a and d are non-polar, which means that each helix has a hydrophobic strip. These hydrophobic strips associate with one another.
- Because the 3.5-residue repeat in α keratin is slightly smaller than the 3.6 residues per turn of a standard α helix, the two keratin helices are inclined about 18° relative to one another, resulting in the coiled coil arrangement

in a coiled coil, each alpha helix is _____1____handed, and the coiled coil itself is _______2____handed
- right
- left
E and G are involved made up of oppositely charged amino acids and are involved in ionic interaction at the right pH. What does this do?
The ionic interactions cause the polarity to be cancelled out which leads to stability, because there will be no polar groups available for interactions outside of the coiled coil.
these ionic interactions are aka “salt bridges”

What’s the hydrophobic pocket and how does that relate to the “knobs in hole” model?
(on coiled coil)
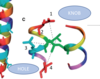
The hydrophobic pocket is the region created by the hydrophobic side chains on “a” and “d” where the 2 helices come together. This pocket is the place of insertion for the “d” amino acid, leading to the knobs in hole model, where “d” is the knob and the hydrophobic pocket is the hole.

Describe each level of structure of a mammalian hair
- The N- and C-terminal domains of each polypeptide of an alpha helix facilitate the assembly of coiled coils (dimers) into protofilaments (using ionic interaction?), each protofilament containing several coiled coils, arranged head to toe and in parallel lines. 2 protofilaments constitute a protofibril.
- Four protofibrils constitute a microfibril, which associates with other microfibrils to form a macrofibril.
- A single mammalian hair consists of layers of dead cells, each of which is packed with parallel macrofibrils.

quarternary structure involves which types of interactions?
all the same interactions as were present in tertiary, disulfide bonds, Van der Walls, and ionic bonds, but involves interactions between multiple polypeptide chains
what is the simplest possible type of protein in quarternary structure?
dimer
what are the 2 types of proteins in the human body
fibrous
- alpha karatin
- collagen
globular
- transmembrane protein
- hemoglobin
- myoglobin










