Exam 1 Reactions Flashcards

Br2
—————>
hv
tertiary radical

NaOMe (or STRONG BASE)
—————————————–>
Zaitsev Eliminatinon reaction

HBr
———————>
ROOR
Anti-markovnikov radiacal addition with
peroxide as a catalyst

HBr
————————>
Markovnikov addition
`

OsO4
————————–>
NMO
Syn Dihidroxylation
syn addition

1) RCO3H or mCPBA
————————->
2) H30+
Anti-dihydroxylation
anti-addition

Br2
—————->
H20
Halohydrin formation
anti-addition
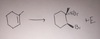
Br2
dihalogenation
anti addition, Sn2-like reaction

H2
————————->
Pt
metal catalyzed Hydrogenation
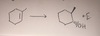
BH3
———————–>
H2O2, NaOH
hydroboration / oxydation
H OH anti-markovnikov Syn-addition
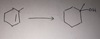
H3O+
——————->
acid catalyzed hydration
H OH markovnikov

1) xs NaNH2
———————>
2) H2O
strong base pulls off bromine

xs NaNH2
————————>
H2O
strong base

HBr
Markovnikov addition

HBr
Markovnikov addition

H2SO4, H20
————————–>
HgSO4, NaOH
enol > ketone

9-BBN
————–>
H2O2, NaOH
9-BBN is a source of boron; this is a hydroboration reaction with a new source
enol>aldehyde

Br2
——————>
anti-addition

xs Br2
——————>

1) O3
————————>
2) H2O
Ozonolysis

1) NaNH2
——————–>
2) MeI (CH3I)

H2
————–>
Lindlar’s Catalyst
(The “C” stands for -cis)

1) Na
————–>
2) NH3 (l)
* -trans* addition

H2
——————>
Pt

NBS
——————>
hv
Radical addition of bromine accross 2 resonance structures


