Exam 1 Muscle Contraction Flashcards
Of what are muscles made? What is the relationship between myofibrils and sarcomeres? What is the sarcomere anatomy (eg bands and lines)?
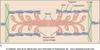
Muscles are made of made of bundles of ‘muscle fibers’, which are made of groups of single muscle cells- the cells are made of myofibrils. Myofibrils are built around the sarcomere. Sarcomeres are made of overlapping thick and thin filaments. The thick filaments are myosin, the thin filaments are actin. Looking at the expanded sarcomere, the space where the myosin does not overlap is the I band. Within the I band is the Z line, from which the actin filaments project. The A band is the length of myosin. With the H zone being the middle where the actin does not reach.
What are titan molecules? Why is it important for the muscle to be “springy”?
Titin molecules: these extend from the Z disc to the M line where it associates with the myosin thick filament. The molecule is “springy”, holds the myosin and actin filaments in place as needed to keep the sarcomere functional.
Describe the (overall) sliding filament mechanism by which muscle contraction is produced.
Muscle contraction is produced by the “sliding filament mechanism”. The myosin filaments interact with the actin filaments in such a way that the myosin grabs and pulls itself along the actin. As the muscle contracts, the myosin pulls itself along the actin, resulting in the actin filaments becoming progressively more overlapped.
Describe the anatomies of myosin, actin, and tropomyosin.
The myosin filament is made of multiple myosin molecules- each myosin molecule is made of heavy (MW 200,000) and light (MW 20,000) chains, and has a tail and a head. The tail is two heavy chains spiraled together and folded at the top end to make two ‘heads’. Each head gets two of the light chains. A myosin filament will have a couple of hundred or so of these molecules. The tails bundle together, with the heads extending outward. The extending section of tail and head is the “cross bridge”, which is flexible at 2 points, where the tail exits the body, and at the start of the head. The cross bridges extend from the filament body in a spiral pattern. The head, as we’ll see, functions as an ATPase enzyme, meaning it cleaves ATP for energy. Actin structure; actin filament is made of two strands of F-actin molecules (light band) and two strands of tropomyosin (darker bands). Along the F-actin molecules are ‘active sites’. Attached to the tropomyosin is the troponin complex, made of three protein subunits; troponin I, T, and C: the I has an affinity for actin, T for troponin, and the C for calcium.
Describe the anatomy of the SR. What is the main function of the SR in regards to muscle contraction?
Sarcoplasmic-reticulum system surrounds the myofibrils. Muscle action potentials start at the surface. For the excitation to travel deep into the muscle, there are transverse tubules (T tubules). These small tubes run transverse to the fibrils and branch to form planes of tubules in the myofibrils. The action potential that starts on a muscle membrane can travel along these tubules into the muscle. Note the SR is made of ‘terminal cisternae’, which are open spaces abutting the T-tubules, and longitudinal tubules which surround the surface of the myofibrils. The SR acts as a reservoir of Ca2+ for intracellular signaling.
How are actin and myosin arranged relative to one another in a sarcomere? How does this change in regards to a muscle being in a relaxed state vs contracted? What is required for contraction to occur? What controls cross bridge attraction to actin? Explain.
As we had seen, in the sarcomere, actin and myosin overlap. In the relaxed state, the myosin heads do not interact with the actin (specifically, the myosin heads don’t interact with the active sites on actin). For contraction to occur, the myosin heads (cross bridges) become bound to the actin at the ‘active sites’. Through a mechanism not entirely understood, the bound cross bridge goes through a conformational change that causes the head to tilt towards the arm, pulling the actin filament with it- the ‘power stroke’. The effect is to bring the Z lines closer together, and make the H zone smaller. The attraction of the cross bridge to actin is controlled by calcium. At low Ca2+, myosin/actin binding sites are blocked by tropomyosin. When elevated, Ca2+ binds to troponin c, troponin complex moves tropomyosin (or rather goes through a confirmation change) to expose myosin binding sites. Myosin then binds to and pulls actin towards center of sarcomere, promoting contraction.
Compare and contract thje effect of low [Ca2+] and elevated [Ca2+] on muscle contraction. Which direction does myosin pull actin in order to promote contraction?
At low Ca2+, myosin/actin binding sites are blocked by tropomyosin. When levels of Ca2+ are elevated, Ca2+ binds to troponin C, troponin complex moves (confirmation change) tropomyosin (to actin groove) to expose myosin binding sites. Myosin then binds to and pulls actin towards center of sarcomere, promoting contraction.
What are the steps of cross-bridge cycling? What is occurring in order in order for this to earnt he name “sliding filament theory”? How does this apply to rigor mortus?
In some sources this process is called “cross-bridge cycling”. 1. In low Ca2+, relaxed state, myosin cross bridge is energized by partial hydrolysis of ATP. 2. Action Potential in muscle induces Ca2+ release from SR, myosin binds to actin- ‘ratchet’ action of myosin head pulls actin towards center of sarcomere- ADP & phosphates released. 3. Binding of ATP to myosin decreases affinity for actin, detachment occurs 4. Return to start of cycle, partial hydrolysis of ATP energizes myosin bridge. This action causes the thin filaments to slide past the thick filaments, hence the name “sliding filament theory”. Without ATP, the cycle stops at stage 3, in the contracted state. This produces “rigor mortus” after death because the body is no longer producing ATP.
During muscle contraction, where does the Ca2+ come from and how is it controlled?
There is a gap between the T-tubule and the SR. A SR Ca2+ channel protein (known as the ryanodine receptor) extends into the gap, and abuts a voltage-gated Ca2+ channel in the t-tubule (known as the dihydropyridine receptor). With depolarization, the T-tubule voltage-gated Ca2+ channel undergoes a configuration change which in turn causes a reconfiguration in the SR Ca2+ channel. This causes the SR Ca2+ channel to open, releasing Ca2+ from the SR into the cytosol. This Ca2+ from the dihydropyridine receptor is returned to the SR via a Ca2+ pump known as SERCA . SERCA: sarco/endoplasmic reticulum Ca2+ ATPase.
How does an action potential start in a muscle? Overview.
An impulse will start in the motor cortex, travel down the spinal cord, and at the appropriate level will synapse on a motor neuron in the spinal cord. That neuron will project out the ventral horn of the cord and proceed to the muscle. The neuron projecting from the brain is the upper motor neuron, while the neuron projecting from the spinal cord is the lower motor neuron (the alpha motor neuron). At the neuron-muscle connection is a specialized type of synapse known as a motor end plate, or neuromuscular junction. Acetylcholine is released from lower motor neuron to evoke PSPs (post synaptic potentials) and action potentials in muscle.
What is meant by the neuromuscular junction being a “chemical synapses”?
The neuromuscular junction is a “chemical synapses”. What this means is that the pre and post synaptic membranes are separated by a space (“a synaptic cleft”), and a chemical signal (“a neurotransmitter”) is released from the pre-synaptic membrane, crosses the synaptic space, and binds to receptors on the post synaptic membrane. The neuromuscular junction contains invaginations or clefts in the post-synaptic membrane. Neurotransmitter receptors are concentrated at the top.
Describe the sequence of events after an action potential travels down the alpha motor neuron.
- An action potential travels down the alpha motor neuron (or lower motor neuron), comes to the pre-synaptic membrane. There, voltage-gated calcium channels (associated with the “dense bar” structure) open. 2. Inside the pre-synaptic terminal are vesicles filled with neurotransmitter- in this case Ach. The influx of Ca induces the vesicles to fuse with the pre-synaptic membrane, releasing the neurotransmitter into the synaptic cleft or space. 3. The transmitter (Ach) then binds to receptors on the post-synaptic membrane.
Describe the flow of events during the process of synaptic transmission at the neuromuscular junction. What important principle of physiology does it demonstrate? Possible pharmacological interventions?
- The muscle post-synaptic membrane forms a trough, with deep folds. The receptors are in the troughs, actually at the mouth of the trough. 2. The vesicles contain acetycholine, which is synthesized in the cell. Choline is taken in from extracellular space, and is combined with acetyl-coenzyme A to form acetylcholine. This reaction is catalyzed by the enzyme choline O-acetyltransferase 3. Ach is released into cleft, binds receptors on post-synaptic membrane. There are usually several types of receptors for a given transmitter, each with a different distribution and function. 4. At neuromuscular junction, the receptors are nicotinic Ach receptors. The transmitter is quickly released from the receptor and is degraded by AChE (acetylcholinesterase). This demonstrates an important general principle of physiology- if something is activated or turned on, there has to be a mechanism for turning it off. Also, note that every step in the process of synaptic transmission is a window of opportunity for pharmacological intervention.
Describe the NMJ’s ACh receptors (where are they located, how are they gated, how do they work?) What is the purpose of the negative charges in the mouth of the channel? How can the muscles be made less excitable?
The ACh receptors are in the top of the post-synaptic fold. These are ligand-gated ion channels in that ligand binding- that is acetylcholine binding- causes a configuration change which causes opening of the channels and allowing Na to enter. This increases the Na permeability, which will drive the cell towards the Na equilibrium potential. The Na equilibrium potential is positive relative to the resting potential, so the effect is to depolarize the cell. Note the negative charges in the mouth of the channel keep negatively charged particles from slipping through. If the depolarization is sufficient, then voltage-gated Na channels open and an action potential is evoked. In healthy individuals, this is almost always the case: the amount of transmitter released is more than enough to evoke a depolarization in the muscle membrane sufficient to bring the membrane to threshold and evoke an AP. Can treat muscles to make it less excitable: with curare to block Ach receptors and botulinum toxin (eg Botox), which blocks transmitter release.
What causes Myasthenia Gravis? How does its diagnostic test demonstrate how AchRs work?
Myasthenia Gravis: The idea that it was an autoimmune disease began in the 1950s, but was reinforced in the early 1970s when a group trying to raise antibodies to AChR immunized rabbits with AChR from electric eels. The rabbits developed muscle weakness which responded to cholinesterase inhibitors- this paralleled the clinical response of human patients and brought attention to the autoimmune theory for MG. This was reinforced after AChR antibodies were found in pts’ blood. -chronic autoimmune disease, most common ‘primary disorder of neuromuscular junction’ -characterized by fatigue and skeletal muscle weakness, especially face, neck. Weakness worsens with activity, improves with rest. Usually involves muscles controlling eye/eyelid movements, facial expression (loss of expression), chewing, talking (slurred speed), swallowing A common diagnostic test is to administer an acetylcholinesterase inhibitor, if symptoms are relieved then strong evidence for MG.
Compare and contrast fast (6) and slow twitch (7) muscle fibers. What determines fast/slow properties?
Fast-twitch muscle fibers: Large diameter, high glycolytic/low oxidative capacity (makes muscle easily fatigued). Anaerobic (pale color). Recruited last. SERCA activity higher, faster relaxation. ’Fast-twitch’ myosin. Slow-twitch muscle fibers Small diameter, high oxidative/low glycolytic capacity (allows fatigue resistance). Higher surface/volume ratio allows easier O2 uptake. High myoglobin, mitocondria, vascularization gives reddish color. Contracts at lower Ca2+ levels. ‘Slow-twitch’ myosin. Fast/slow properties appear to be determined by α-motor neuron activity.
What are the divisions of skeletal muscle twitches? How does alpha motor neuron activity determine fast/slow properties? How does chronic stimulation/decreased stimulation of motor units affect their characteristics? To what does a “motor unit” refer?
Skeletal muscle can be divided into fast, intermediate, and slow twitch. Fast/slow properties appear to be determined by alpha motor neuron activity: neuron from slow motor unit crossed innervated to fast muscles causes muscles to take on slow characteristics & vice versa also holds. Appears to be produced by a pattern of electrical activity. Artificial chronic stimulation of fast-twitch motor unit promotes synthesis of slow-twitch myosin. Decreased stimulation of slow twitch unit promotes adoption of fast twitch characteristics. Mechanism unclear (proposed to be related to chronic Ca++ levels). “motor unit” refers to an alpha motor neuron and all the muscles it innervates.
Compare/contrast the contraction of slow and fast muscle units. How is force of contraction increased?
To increase force of contraction, recruit more muscle units. Slow muscle units get recruited first, slow muscle alpha MN (the neuron innervating a muscle unit) has a small diameter which makes it more excitable with a lower threshold. These tend to be smaller motor units, good for fine control. Fast muscle units get recruited later, the alpha MN has larger diameter, higher threshold. These tends to have larger motor units, larger forces are generated.
Explain spatial summation and temporal summation.
Spatial Summation: increasing contraction force by recruiting more motor units Temporal Summation: increasing contraction force by frequent stimulation of muscle. Tetanus occurs when stimulation is so frequent muscle is not allowed time to relax between contractions.
What are the type basic types of smooth muscle? Describe them.
Smooth muscle two basic types; multi-unit smooth muscle and unitary smooth muscle. Unitary or single unit smooth muscle: These are masses of fibers which contact each other making a single unit. They are physically connected so that they contract together, and are connected electrically via gap junctions and form a syncytium. It is found in the visera of the body, lining the gut, lining the uterus, blood vessels. Multi-unit: composed of discrete separate muscle fibers which operate independently. These are innervated by single neurons (like skeletal muscles) and are largely controlled by neurons. Examples are the muscles controlling the irus and the muscle that causes erection of hair.
Explain the main four ways in which smooth muscle contraction differs from skeletal muscle contraction.
Contraction in Smooth Muscle: smooth muscle has actin and myosin filaments as we saw in skeletal muscle. These muscles do not have the troponin complex however so some of the physiology of contraction differs. 1. Smooth muscle is smooth, not striated, actin filaments are attached to “dense bodies”. Interspersed among the actin are much larger myosin filaments. 2. Slow cycling: cycling of myosin cross bridges is much slower than in skeletal muscle. The cross-bridges (heads) stay attached to the actin for longer periods of time and heads have less ATPase activity. 3. Less energy: SM requires much less energy to maintain comparable tension than skeletal muscle (due to slow cycling). This is important since SM has to maintain tonic constriction over long periods of time. SM can also generate greater force of contraction per unit of muscle than skeletal. 4.Stress/Relaxation: SM is typically around hollow organs. These organs can expand and contract, and the SM will (after a brief period of time) stretch or contract to maintain constant pressure.
Explain the events of smooth muscle contraction (4 steps). How is it different than skeletal muscle contraction?
Smooth muscle contraction: the role of Ca2+; In lieu of troponin, Ca++ is regulated by calmodulin. 1. Ca++ enters the cell, binds with calmodulin. 2. Calmodulin-calcium binds with and activates myosin light chain kinase (MLCK). Remember kinases phosphorylate things. 3. Myosin is made of 2 heavy and 2 light chains, actin has no troponin; the binding inhibited by the light chain. Regulatory light chain of myosin head is phosphorylated (by the kinase) and binds to actin filament and goes through cycling, causing the muscle to contract. 4. Myosin phosphatase (in the cytosol) splits the phosphate from the regulatory chain, the head detaches from the actin, cycling stops and the muscle relaxes.
BRIEFLY explain the events of smooth muscle contraction and relaxation.
Ca++-Calmodulin binds and activates MLCK. This phosphorylates the myosin head, which allows binding to actin and contraction is induced. To relax, Ca++ is reduced and myosin phosphatase removes the phosphate from the myosin light chain, the myosin head detaches from actin, and the muscle relaxes.
Explain the role of Ca2+ in smooth muscle contraction (4 steps).
Increased intracellular Ca2+ induces contraction, but smooth muscle does not contain troponin (which was activated by Ca2+ in skeletal muscle). Instead, Ca++-dependent phosphorylation by myosin light-chain kinase allows myosin/actin interaction, largely resembles cross-bridge cycling thereafter. 1. Calcium ions bind with the regulatory protein calmodulin. 2. Calmodulin-calcium activates myosin kinase. Kinases are enzymes which phosphorylate things, meaning add phosphate groups. 3. Myosin kinase phosphorylates one of the light chains of each myosin head (the regulatory chain). This allows the myosin head to bind with the actin filament and the ‘cross-bridge cycle’ is executed. 4. This process reverses in low Ca2+. Phosphatases undue the phosphorylation of kinases, and the reversal of the phosphorylation of the regulatory chain requires the action of myosin phosphatase. Ca++ is lowered through- SERCA, 3Na-1Ca antiporter, and the sarcolemma Ca-pump.