Exam 1 Flashcards
(124 cards)
Describe chemical components, structure, and function of cell membrane
Composed of lipids, proteins, and carbohydrates. Function is to separate and maintain chemical environments.

Cellular membrane lipids
majority are phospholipids, amphipathic

Cholesterol function in cell membrane
affects fluidity and permeability

Transport through lipid bilayer
small hydrophobic molecules and gases can get through (O2, CO2, N2, benzene). Large and charged ions cannot (H+, Na+, glucose)

Lipid rafts
Interactions between specific lipids (cholesterol, saturated lipids, and glycosylated lipids) in plane of cell membrane drive formation of lipid rafts. They are enriched in saturated phospholipids, sphingolipids, glycolipids, cholesterol, lipated proteins, and GPI anchored proteins. They segregate specific elements in order to regulate their interactions with other cell membrane components.
Carbohydrate molecules and cell surface
Function to protect, cell to cell interaction (ganglioside GM1 acts as a cell surgace receptor for the bacterial toxin that causes diarrhea of cholera)
Functions of membrane proteins
receptors, transport-channels, enzymes, structural
Cytoskeleton function and components
filamentous scaffold of proteins that contribute to the internal organization of cytoplasm and stabilization of cell membrane. Consists of microtubules, actin filaments, and intermediate filaments. Actin filaments and microtubules generate forces to drivve cell shape and motility.

What keeps cells shape
Proteins
Types of SIGNAL molecules
steroids, polypeptides, proteins
Examples of intracellular receptors
steroid and thyroid hormones.
List 3 classes of cell surface receptors.
- Ion channel linked
- G protein-linked
- Enzyme-linked
Ion channel linked cell surface receptors
Ach receptor at neuromuscular junction, neurotransmittter receptors, serotonin, GABA, and glycine. Permeable to Na+, K+, and Ca+

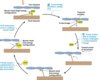
List components of G protein linked receptor signaling machinery
3 subunits- alpha, beta, and gamma. (beta and gamma form stable complex, BYsubunit). Upon stimulation, GPCR undergoes conformational change that expose intracellular sites that activate G protein. This catalyzed dissociation of GDP bound to the Ga subunit and its replacement with GTP, leading to dissociation of Ga from BY subunit. Ga-GTP and GBy-subunit complexes are freely able to active downstream effectors. Targets of dissociated components are enzymes or ion channels in plasma membrane, and they relay signal onward.

Give examples of chemicals that activate stimulatory and inhibitory G protein subunits.
G1 anf Gs regulate activity of adenylate cyclase, altering cAMP levels.
Gs stimulates adenylyl cyclase, associated with adrenaline, B1, glucagon, and ACTH.
Gi inhibits adenylyl cyclase lowering level of cAMP and it is activated by receptor for somatostatin, muscarinic receptor.

Endoplasmic reticulum
Rough (ribosomes)- protein synthesis
Smooth- lipid synthesis
Golgi apparatus
Substances synthesized in ER get transported here for further processing and distribution
3 types of endocytosis
phagocytosis, pinocytosis, and receptor mediated endocytosis
Diffusion vs Active transport
Diffusion: movement is always down concntration gradient. Active movement against concentration gradient, requires ATP
Differentiate Simple and facilitated diffusion
Simple: Through membrane or channel proteins (ion channels), NOT carrier proteins. lipophilic molecules, water, small molecules (urea). Rate inreases with increasing concentration gradient.
Facilitated: SPECIFIC CARRIER PROTEIN helps transport substances across membrane down conc gradient. Saturation kinetics (rate will not necessarily increase with increase in concentration gradient). ex.) glucose, amino acids, chloride bicarbonate transport.
Factors that affect rate of diffusion
Concentration, membrane electric potential, pressure.
Osmosis
flow of water across semi permeable membrane from solution with low solute concentration to one with high solute concentration
Primary active transport example
Na/K ATPase- 3 Na out, 2 K in. establishes negative voltage inside the cells. Activated by insulin and beta 2 adrenergic agonists.
Seconday active transport example
glucose/na, di and tripeptides, H+ and HCO3-