Erhand Hohenester - Cell Signalling Flashcards
(167 cards)
What is one way of conceptualize/understanding cell signalling?
One way to conceptualize cell signalling is to visualize a cell as device that converts inputs signals into output signal (in response to the input)
Focus of lecture serious - Input signals from other cells
Output – very varied
Not a unidirectional process – feedback/communication between output & inputs

What type of signalling does this lecture series focus on?
Main focus – inter (between) cellular signalling
Involves a cell releasing an extracellular signalling molecule (peptide/protein/hormone) that cannot pass through membrane unaided.
Hence, it binds to a receptor molecule - yields a change in the receptor which leads to a sequence of intracellular signalling events, ultimately influencing an effector protein
Outline the difference between receptor activation and Signal transduction/downstream signalling?
Receptor activation - refers to information being transferred across the P.M
Signal transduction/downstream signalling - refers to transfer of information within the cytosol and nucleus
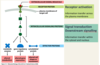
What are the two main families of receptors which will be discussed?
- G protein-coupled receptors
- Receptor tyrosine kinases
What’s not included: Notch, Wnt, Hedgehog, NFκB, cGas-STING
What’s also not included: Signalling pathways in bacteria and plants
What are the different types of intercellular signalling?
- Contact dependent – signalling cell physically targets the target cell via two transmembrane cell membrane proteins widespread but common in the Immune system
- Paracrine - local mediator meaning that it interacts with neighboring cells
- Synaptic - neuron releases neurotransmitter – released into synapse
- Endocrine - can interact with cells over large distances as the hormone/signalling molecule is released into the blood stream
This lecture series will focus on Paracrine and endocrine intercellular signalling

What is the difference between paracrine and endocrine?
Paracrine & endocrine – Both involve the release of soluble mediators by signalling cell which interact with a target cell, but the difference is distance that the signalling molecule can travel and effect
Paracrine - Short distances effecting neighbouring cells
Endocrine - Longer distances
What is autocrine signalling?
Autocrine - form of paracrine signalling –> cell is both a signaling cell and target cell
What type of signalling are growth factors, cytokines and hormones involved in?
- Growth factors - Paracrine & Endocrine
- Cytokines - Paracrine & Endocrine
- Hormones - Endocrine
Outline what the following types of signlling molecules are/where they are produced/what is their role?
- Cytokines
- Chemokines
- Hormones
- Growth Factors
Types of signal molecules
- Cytokines are secreted mainly by immune cells and modulate the immune response.
- Chemokines are a subset of cytokines that function as chemoattractants.
- Hormones are produced by endocrine glands and distributed by the bloodstream. They can be small organic molecules (adrenaline), peptides or proteins, and have a wide variety of effects.
- Growth factors stimulate cell growth, proliferation and differentiation. Some cytokines and hormones act like growth factors.
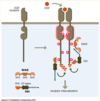
Do most signalling molecules pass straight through the membrane or interact with PM receptors?
Most molecules are hydrophilic meaning they need a cellular receptor
Example – Beta-adrenergic receptor –> epinephrine binds resulting in conformation change – change intracellular domain which initiates as signal transduction cascade

Do hydrophobic molecules also bind to receptors?
Most small hydrophobic molecules can diffuse through the P.M., allowing them to bind to intracellular receptors instead
e.g. Steroid & sex hormones

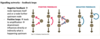
Do cells normally respond to singular or a multitude of singalling inputs?
Animal cells respond to multiple signal inputs – never just receive one signal –> coordination between a multitude of signals
Act like a control panel receiving a mutlitude of signals, from which it decides it’s next step/action
Signal integration - The process of responding correctly to a number of different signals –> receive multitude of signals and respond with the appropriate output
Example attached is a simple representation of this concept

Can the sam signalling molecule elicit different responses?
YEAHHHH BOI, context is important as it can influence/alter the effect of signalling molecules, this case cell type influencing the ACh effects

What ‘changes/modifications’ are commonly used to alternate between active and inactive states in a signalling cascade?
Changes in state link signal inputs to outputs
Signalling information is transduced through the cell and goes through a signalling nodes which switch from an inactive to active state. This switch from off to on can be as a result of…
- Conformational changes
- Addition or removal of post-translational modifications
- Formation or dissociation of complexes
- Changes in subcellular localization

What effects do PTMs have on the states of signalling molcules/nodes?
Effects of post-translational modifications
Changes in state are often associated with PTM as it can alter…
- Conformation
- Promote partner binding
- Prevent protein binding
- PTM can influence localization – nuclear vs. cytoplasmic
- Proteolytic stability

What writers, readers and erasers system when discussing PTM?
Most common PTM is phosphorylation –> takes place on Tyrosine, serine or threonine
- Kinase adds phosphate (Writer)
- Phosphatase removes phosphate (Eraser)
- In on the on state, molecules bind (reader) to the activated form of the protein and mediate the output/response
System known as the writer, reader and eraser system
This system is not limited to phosphorylation but also applies to other PTM – Acetylation, ubiquitination

How many different kinases & phosphatases are known to exist in the human genome and what is their conserved structure (Kinase)?

How is kinase activity regulated?
Kinase activity is regulated by conformational changes and phosphorylation of the activation loop
Kinase is regulated by phosphorylation itself – autophosphorylation
- Inactive state – activation loop (orange) blocks the binding site for ATP + C-helix (purple) is in up conformation inhibiting the formation of Lys-Glu salt bridge
- Active state – Loop undergoes conformational change that opens up the active site for ATP and substrate binding + C-helix (purple barrel) switches from the ‘up’ to ‘down’ conformation which forms a salt bridge (Lys-Glu) which is required for catalytic activity
As long as the Loop is phosphorylated the kinase remains in its active state until a phosphatase comes in to remove them

Why are many anti-cancer drugs protein kinase inhibitors? Provide one example.
Many kinases become mutated and overexpressed in cancer cells - driving forward cell growth and progression. Thus, making it an attractive drug target
Imatinib (GleevecTM, GlivecTM) is an ATP-competitive inhibitor targeting the Bcr-Abl fusion protein (fusion protein caused by chromosomal translocation - Abl is a tyrosine kinase) encoded by the Philadelphia chromosome (abnormal Chromosome 22) – constitutively active (constantly)
Imatinib binds to between the Lobes and blocks action - was developed for the treatment of chronic myelogenous leukaemia and has more than doubled the 5-year survival rate of this disease.

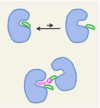
Outline how GTPases are used as molecular switched.

What is one very large obstacle that the cell needs to overcome in order to acheive effective signalling?
Signal transduction takes place in a crowded environment
Cell’s are extremely busy environment - difficult to coordinate these changes of activation and inactivation –> Still one of the big areas of research
How can the formation of signalling complexes increase signalling specificity/overcome cell crowded environment?
Signalling complexes increase signalling specificity

How does multivalency and membrane association help increase signalling specificity and overcome the busy cellular environment?

Is there a high degree of response time variability when it comes to cellular signalling?