Distortion of evidence-based medicine Flashcards
How is the evidence base distroted
- Missing data- publication bias
- Flawed clinical trials
- Others
- Allowing politics to trump evidence
- Regulatory capture
Why does distortion of the evidence base matter
- Clinical decisions are based largely on published data
- Systemic review/meta-analyses key tools
- If the published data are not an accurate reflection of reality then this may lead to
- Decisions that cause avoidable suffering/death
- Wasted resources
- Where prescribers are misled into thinking that newer, more expensive treatments are more effective than older ones
- Where healthcare funding is finite (e.g. everywhere), this deprives patients of other treatments
What is publication bias
- Publication bias is the tendency on the parts of investigators, reviewers, and editors to submit or accept manuscripts for publication based on the direction or strength of the study findings
How does publication bias happen
- Authors are more likely to submit positive (rather than negative or inconclusive) results
- Editors are more likely to accept positive results
- Pressure on academics to publish compounds the problem
- Negative or inconclusive results often remain unpublished
Impact of publication bias on meta-analyses

Publication bias: Reboxetine
- NA reuptake inhibitor
- Used in unipolar depression
- Marketed by Pfizer as Edronax
- Very common adverse events include Nausea and insomnia
Reboxetine- Benefits

Reboxetine- harms

Publication bias in antidepressant trials

Publication bias in reporting of adverse events as well as treatment outcomes

Publication bias is compounded by industry funding of trials
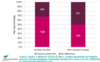
Outcome reporting bias
- The selective reporting of some outcomes but not others, depending on the nature and direction of the results
Outcome reporting bias
Flawed trials: external validity of RCTs in asthma

Flawed trials: impact of atypical study populations on cost-effectiveness decisions

Manipulation of trials: comparing your new drug with something rubbish
- Comparing your drug with placebo when effective treatments are already available
- Psychopharmacology
- Paroxetine versus amitriptyline BD
- Atypical antipsychotics versus haloperidol 20mg
- Newer atypicals vs risperidone 8mg
Flawed trials: Stopped to short

Flawed trials: Use of surrogate outcome measures- Torcetrapib

Flawed trials: Others
- Trials that are too small
- Trials that don’t measure drop-outs
Tamiflu
- Oseltamivir
- Manufactured by Roche
- Neuraminidase inhibitor (Anti-viral) used for treatment and prevention of influenza A + B
1997-99: Emergence of pandemic flu as a threat
- May 1997- 1st documented human cases of avian influenza H5N1 occurred in Hong Kong.
- April 1999- WHO publishes its first pandemic influenza plan
- Recommends use of antivirals as prophylaxis
- Written in collaboration with the European Scientific Working Group on Influenza (ESWI) a group ‘funded entirely by Roche and other influenza drug manufacturers’
1999-2002: Approvals and calls to stockpile
- 1999-2000–FDA approvals of Tamiflu for treatment and prophylaxis of influenza
- 2002–EU approval for Tamiflu for treatment and prophylaxis of influenza
- May 2002–WHO calls for nations to stockpile influenza antivirals
2003-05- stockpiling begins
- Aug 2003- the US begins stockpiling Tamiflu (est. spend to date = $1.3 billion)
- March 2005- UK government announces it will stockpile 14 million doses of Tamiflu (Est spend to date= £424 million)
2006: Controversy about effectiveness of Tamiflu gathers pace
- July 2006–Cochrane review finds Tamiflu to be minimally effective with no comparative data regarding its effectiveness in treating avian influenza.“
- Because of their low effectiveness, NIs should not be used in routine seasonal influenza control. In a serious epidemic or pandemic, NIs should be used with other public health measures. We are unsure of the generalizability of our conclusions from seasonal to a pandemic or avian influenza.”


