Diffusion Flashcards
(27 cards)
State Fick’s law.

Draw a small control volume for diffusivity and construct the general mass balance.
See footnote 38.
Simplify the differential equation for transient diffusion using a simalarity variable.
See footnote 39.
Solve the simplified differential equation for transient diffusion.
See footnote 40.
Apply the boundary conditions to the simplified equation for transient diffusion, and change the resulting equation back to the original variables.
See footnote 41.
Derive the equation for mass flux at the surface of a transient diffusion situation.
See footnote 42.
Derive the equation for total mass transfer per unit area for transient mass diffusion.
See footnote 43.
Draw a diagram to represent gas dissolution in a falling film.
See footnote 44.
Draw the control volume for dissolution of gas in a falling film.
See footnote 45.
Construc the mass balance for gas dissolution in a falling film and state the boundary conditions.
See footnote 46.
Simplify the differential equation for mass dissolution through a falling film using a simalarity variable.
See footnote 47.
Solve the differential equation for gas dissolution into a falling film.
See footnote 48.
Apply the boundary conditions to the simplified version of the equation for mass dissolution through a falling film and convert it back to it’s original variables.
See footnote 49.
Derive the equation for the surface flux of dissolution through a falling a falling film.
See footnote 50.
Derive the equation for the total rate of absorption for mass dissolution through a falling film.
See footnote 51.
Draw a diagram for a gas (A) absorbing into a liquid (B) that reacts to the reaction A+B=AB
See footnote 52.
Draw a control volume for gas diffusing into a liquid to a reaction A+B=AB
See footnote 53.
Construct the mass balance for gas diffusing into a liquid with the reacter A+B=AB and state the boundary conditions.
See footnote 54.
Use non-dimensionalised variables to simplify the mass balance for gas diffusing into a liquid with a reaction.
See footnote 55.
What is the Thiele modulus?
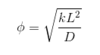
Solve the differential equation in terms on non-dimensional variables for gas diffusion into a liquid with a reaction.
See footnote 56.
Apply the boundary conditions to the equation with non-dimensionalised variables to find the constants and return the equation to the original variables.
See footnote 57.
What happens to the Thiele modulus when reaction rate increases?
It increases
What happens to the Thiele modulus when diffusion rate increases?
It decreases



