Chemistry Flashcards
What are the pH of acids?
Acids = 0 - 6
What are the pH of bases?
Bases = 8 - 14
Are metal hydroxides acids or alkalis/bases?
Bases
Are metal carbonates acids or alkalis/bases?
Alkali/bases
Are metal oxides acids or alkalis/bases?
Alkali/bases
What is the difference between an alkali a base
Bases are solid, alkalis are soluable bases
Define amphoteric
Can act as an acid and a base
What is a general equation for a neutralisation reaction?
Acid + Alkali –> Salt + Water
Write balanced symbol equations for the neutralisation reactions between hydrochloric acid and sodium hydroxide.
HCl + NaOH –> H₂O + NaCl
Acid is a ___ donor
Proton (H+)
Alkalis produce what ion in solution
OH-
Alkali is a ___ acceptor
H+
Acid creates ___ in water
H+
Bases ______ an acid
Neutralises
All alkalis are ___
Bases
What is a nucleophile?
An electron rich species, e.g. a species with a long pair or a negative charge
What is an electrophile?
An electron poor species, e.g. positively charged species
Outline the mechanism for the electrophillic addition of HBr to ethene

State uses for alumina (Al2O3)
The major uses of aluminium oxide is in refractories, ceramics, polishing and abrasive applications.
Describe what happens to ions (Al3+ and O2-) when Al2O3 is electrolysed
Al3+ gains electrons and forms pure Aluminium (Reduction), O2- looses electrons and forms Oxygen gas (Oxidation)
State half equations for the reactions at the electrodes
Anode) 2O2- —> O2 + 4e- Cathode) Al3+ + 3e- —> Al
Describe the reactions at the electrodes as oxidation/reduction
The reaction at the anode is Oxidation (losing electrons), and the reaction at the cathode is Reduction (gaining electrons)
Explain why electrolysis of alumina is expensive
It’s expensive due to the large amount of electricity required to keep the alumina (bauxite) molten to allow the electrolysis to continue 24/7, 365
State uses of titanium
Titanium metal is used as an alloying agent with other metals (e.g. aluminium). Alloys of titanium are used in aerospace, aircraft and engines due to the strong, lightweight, temperature-resistant properties.
Describe the steps in the Kroll process
Step 1) Titanium (IV) dioxide, coke (form of carbon) and chlorine are heated together at 900c to form Titanium (IV) chloride. TiO2(s) + 2C(s) + 2Cl2(g) —> TiCl4(g) + 2CO(g) Step 2) Magnesium is used as a reducing agent, which reacts with the chlorine to remove it from the titanium, forming pure titanium metal, and magnesium chloride liquid. TiCl4(g) + 2Mg(s) —> Ti(s) + 2MgCl2(l)
Explain why the Kroll process is expensive
The process required very high temperatures to break the strong bonds in titanium. It’s very labour intensive. Magnesium (or other metals used) is a very expensive metal, and is used in large quantities to obtain the pure titanium.
State the definition of a transition metal
forms one or more stable ions which has incompletely filled d orbitals
State characteristics of transition metals
-have an incomplete D subshell -have variable oxidation states -forms complex ions -catalysts -coloured componds
State what a complex ion is
Complex ion - a positively charged ion that is surrounded by a number of molecules that are oppositely charged.
State what a dative bond is
Dative bond - when both electrons are donated by one molecule to form a covalent bond.
State what a ligand is
Ligand - a molecule with a lone pair of electrons e.g. water or ammonia.
State what a catalyst is
Catalyst - speeds up chemical reactions
State how a catalyst affects the rate of a reaction
catalyst lowers the activation energy by providing a different pathway
Explain why transition metals make good catalysts
-variable oxidation rates -can be oxidised and reduced (change their oxidation state) -lower thr activation energy by providing an alternitive route
Explain why transition metals form complex ions
They are small with high charge to size ratios. They have empty ‘s’ and ‘d’ orbitals to accept lone pairs from ligands.
Describe the stages of the Contact Process
Stage 1: sulfur dioxide is made (mixed with excess air) Stage 2: sulphur trioxide is made SO2 + V2O5 = SO3 + V2O4 Stage 3:sulphur trioxide is converted into sulphuric acid V2O4 + 0.5 O2 = V2O5
Describe the use of Iron in the Haber process
It makes ammonia.
Whats is left in the solution in the ectrolysis of brine
Na+ and OH- from thr water are left in the soloution, this forms sodioum hydroxide
What are uses of sodium hydroxide?
the purification of bauxite to make aluminium oxide (alumina) as a part of the manufacture of aluminium making paper - the sodium hydroxide helps break the wood down into pulp making soap - sodium hydroxide reacts with animal and vegetabl fats and oils to make compounds, such as sodium stearate, that are present in soap making bleach - bleach is formed when sodium hydroxide and chlorine react together in the cold; it is a mixture of sodium chlordie and sodium chlorate (I) solution (NaClO)
In a diaphragn cell why can brine pass from one side to the other but gases can’t?
The diaghragm dividing the cell is porous
How is the sodium chloride separated from sodium hydroxide in a diaphragm cell?
It is crystallised (less soluble)
In a membrane cell why can only sodium ions move from one side to the other?
The divider is an ion-exchange membrane that only allows positive ions through
How is sodium hydroxide separated from sodium chloride in the membrane cell?
It is already separated as sodium hydroxide is left on the cathode side
What are the pros and conds of diaphragm cell?
Pros: cheap Cons: diaphragm needs replacing regularly, impure NaOH is made and uses slightly more energy
What are the pros and cons of membrane cell?
Pros: Membrane needs little maintenance, pure NaOH formed, slightly less energy Cons: expensive
State uses for the products formed from the electrolysis of brine
Chlorine used as disinfectant and purifier, manufacture of hydrochloric acid and making plastics. Sodium Hydroxide used for processing food products, removing pollutants from water and manufacture of paper. Hydrogen used in the manufacture of hydrochloric acid and potential as a pollution-free fuel
Describe the movement of ions during the electrolysis of brine
Sodium ions will move to the negative electrode (anode) and chloride ions will move to the positive electrode (cathode)
What is produced at the positive electrode for the electrolysis of brine?
Chlorine gas
What is produced at the negative electrode for the electrolysis of brine?
Hydrogen gas
Why is hydrogen produced at the negative electrode in the electrolysis of brine and not Sodium?
Sodium is more reactive than hydrogen so will prefer to lose an electron rather than gain
Write half equations for the reactions at each electrode in the electrolysis of brine
Cathode: 2Cl- –> Cl2 + 2e- Anode: 2H+ + 2e- –> H2
Describe the reactions at the elctrodes in the electrolysis of brine as oxidation/reduction
Cathode: Chloride ions are oxidised (oxidation is loss) Anode: Hydrogen ions are reduced (reduction is gain)
State the general formula for alkanes?
CnH2n+2
What is the molecular formula for methane?
CH4
What is the molecular formula for butane?
C4H10
What is the structural formula for hexane?

Name the following:

2-methyl-propane
Draw a dash/wedge diagram for methane

What is the general formula for alkenes?
CnH2n
Why are alkenes unsaturated compounds?
Because they have double bonds between carbon atoms
Name the following compound

propene
Draw the structural displayed formula for hex-2-ene

Draw the structural formula of cyclopentane

What is a structural isomer?
Same molecular formular but different structural formula (arrangement of bonds)
What is a stereoisomer?
Same molecular formula but different arrangement of atoms in space
Is this cis (Z) or trans (E)

cis (Z)
Is this cis (Z) or trans (E)
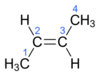
trans
Why does the boiling point of alkanes/alkenes increase with increasing chain length?
Increased surface area, more (stronger) intermolecular forces between chains and therefore more energy required to break them
Why is the boiling point of branched alkanes lower than that of unbranched?
Branched alkanes cannot pack as closely together and therefore the intermolecular forces are weaker and require less energy to break.
What are the products of complete combustion?
carbon dioxide + water
Complete word & symbol equations for combustion of octane
word: octane + oxygen –> cardon dioxide + water symbol: C8H18(l) + 12.5 O2(g) –> 8CO2(g) + 9H2O(l)
State the conditions required for free radical substitution reaction
UV light
Name three steps in free radical substitution reactions
Initiation, Propagation and termination
Describe the three steps in free radical substitution reactions
Initiation - homolytic bond fission of a halogen to form 2 halogen free radicals. ( 2 radicals after arrow) Propogation - steps where one free radical reacts and forms a different free radical. ( 1 radical before arrow, 1 after) Termination - combination of any two free radicals to form a stable product. ( 2 radicals before arrow)
Write equations for the three steps of free radical substitution reactions for a given reaction E.g C2H6 + Br2 → C2H5Br + HBr
Initiation - Br2 → 2Br• Propagation - C2H6+ Br• → •C2H5 + HBr •C2H5 + Br2 → C2H5Br + Br• Termination - 2Br• → Br2 2•C2H5 → C4H10 •C2H5 + Br• → C2H5Br
Explain why there are multiple products of a free radical substitution reaction
Because its a cahin reaction
Outline the steps of free radical substitution reaction for polymers
Initioation: no radicals –> 2 radicals e.g: Br2 –> 2Br• Propagation: Radical + no radical –> Radical + no radical e.g: C2H6 + Br• –> •C2H5 + HBr •C2H5 + Br2 –> C2H5Br + Br• Termination: Radical + non radical –> no radicals e.g: 2Br• –> Br2
Explain the importance of cracking alkanes
To make smaller, higher demand alkanes.
Does the diagram show an endothermic or exothermic reaction?

Exothermic
Does the diagram show an endothermic or exothermic reaction?

Endothermic
Which type of reaction leads to energy being dissipated to the surroundings?
Exothermic
Which type of reaction absorbs energy from the surroundings?
Endothermic
For which type of reaction would you observe a drop in temperature?
Endothermic
For which type of reaction would I observe an increase in temperature?
Exothermic
What is the definition of enthalpy?
The amount of energy transferred per amount of substance
What is the unit for enthalpy?
J/mol or kJ/mol
What is the equation for calculating energy change?
Energy transferred = mass x Specific Heat Capacity x temperature change
For a endothermic reaction would enthalpy change be negative or positive?
Positive
For a exothermic recation would enthalpy change be negative or positive?
Negative
Define enthalpy of formation
The energy change when one mole of substance is made from its elements in their standard form under standard conditions
Define enthalpy of combustion
Energy change when one mole of substance burns completely in oxygen under standard conditions
Define enthalpy of neutralisatin
the enthalpy change when solutions of an acid and an alkali react together under standard conditions to produce 1 mole of water.
Define Hess’ Law
The enthalpy change for any reaction is independent of the route taken


