Chapter 8. Enzymes: Basic Concepts and Kinetics Flashcards
(27 cards)
Question 8.1
Raisons d’être. What are the two properties of enzymes that make them especially useful catalysts?
- Rate enhancement and substrate specificity.
Question 8.2
Partners. What does an apoenzyme require to become a holoenzyme?
- A cofactor.
Question 8.3
Different partners. What are the two main types of cofactors?
- Coenzymes and metals.
Question 8.4
One a day. Why are vitamins necessary for good health?
- Vitamins are converted into coenzymes.
Question 8.5
A function of state. What is the fundamental mechanism by which enzymes enhance the rate of chemical reactions?
- Enzymes facilitate the formation of the transition state.
Question 8.6
Nooks and crannies. What is the structural basis for enzyme specificity?
- The intricate three-dimensional structure of proteins allows the construction of active sites that will recognize only specific substrates.
Question 8.9
Mountain climbing. Proteins are thermodynamically unstable. The ΔG of the hydrolysis of proteins is quite negative, yet proteins can be quite stable. Explain this apparent paradox. What does it tell you about protein synthesis?
- Protein hydrolysis has a large activation energy. Protein synthesis must require energy to proceed.
Question 8.10
Protection. Suggest why the enzyme lysozyme, which degrades cell walls of some bacteria, is present in tears.
- The enzymes help protect the fluid that surrounds eyes from bacterial infection.
Question 8.11
Mutual attraction. What is meant by the term binding energy?
- Binding energy is the free energy released when two molecules bind together, such as when an enzyme and a substrate interact.
Question 8.12
Catalytically binding. What is the role of binding energy in enzyme catalysis?
- Binding energy is maximized when an enzyme interacts with the transition state, thereby facilitating the formation of the transition state and enhancing the rate of the reaction.
Question 8.13
Sticky situation. What would be the result of an enzyme having a greater binding energy for the substrate than for the transition state?
- There would be no catalytic activity. If the enzyme–substrate complex is more stable than the enzyme–transition-state complex, the transition state would not form and catalysis would not take place.
Question 8.14
Stability matters. Transition-state analogs, which can be used as enzyme inhibitors and to generate catalytic antibodies, are often difficult to synthesize. Suggest a reason.
- Transition states are very unstable. Consequently, molecules that resemble transition states are themselves likely to be unstable and, hence, difficult to synthesize.
Question 8.18
Keeping busy. Many isolated enzymes, if incubated at 37°C, will be denatured. However, if the enzymes are incubated at 37°C in the presence of substrate, the enzymes are catalytically active. Explain this apparent paradox.
- The three-dimensional structure of an enzyme is stabilized by interactions with the substrate, reaction intermediates, and products. This stabilization minimizes thermal denaturation.
Question 8.19
Active yet responsive. What is the biochemical advantage of having a KM approximately equal to the substrate concentration normally available to an enzyme?
- At substrate concentrations near the KM, the enzyme displays significant catalysis yet is sensitive to changes in substrate concentration.
Question 8.21
Angry biochemists. Many biochemists go bananas, and justifiably, when they see a Michaelis–Menten plot like the one shown below. To see why, determine the V0 as a fraction of Vmax when the substrate concentration is equal to 10 KM and 20 KM. Please control your outrage.
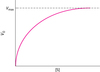
- When [S] = 10 KM, V0 = 0.91 Vmax. When [S] = 20 KM, V0 = 95 Vmax.
So any Michaelis–Menten curves showing that the enzyme actually attains Vmax are pernicious lies.
Question 8.24
Counterpoint. Penicillinase (β-lactamase) hydrolyzes penicillin. Compare penicillinase with glycopeptide transpeptidase.
- Penicillinase, like glycopeptide transpeptidase, forms an acyl-enzyme intermediate with its substrate but transfers the intermediate to water rather than to the terminal glycine residue of the pentaglycine bridge.
Question 8.27
Defining attributes. What is the defining characteristic for an enzyme catalyzing a sequential reaction? A double-displacement reaction?
- Sequential reactions are characterized by the formation of a ternary complex consisting of the enzyme and both substrates. Double-displacement reactions always require the formation of a temporarily substituted enzyme intermediate.
Question 8.29
A tenacious mutant. Suppose that a mutant enzyme binds a substrate 100 times as tightly as does the native enzyme. What is the effect of this mutation on catalytic rate if the binding of the transition state is unaffected?
- The mutation slows the reaction by a factor of 100 because the activation free energy is increased by +11.42 kJ mol−1 (+2.73 kcal mol−1). Strong binding of the substrate relative to the transition state slows catalysis.
Question 8.31
Controlled paralysis. Succinylcholine is a fast-acting, short-duration muscle relaxant that is used when a tube is inserted into a patient’s trachea or when a bronchoscope is used to examine the trachea and bronchi for signs of cancer. Within seconds of the administration of succinylcholine, the patient experiences muscle paralysis and is placed on a respirator while the examination proceeds. Succinylcholine is a competitive inhibitor of acetylcholinesterase, a nervous system enzyme, and this inhibition causes paralysis. However, succinylcholine is hydrolyzed by blood-serum cholinesterase, which shows a broader substrate specificity than does the nervous system enzyme. Paralysis lasts until the succinylcholine is hydrolyzed by the serum cholinesterase, usually several minutes later.
- As a safety measure, serum cholinesterase is measured before the examination takes place. Explain why this measurement is good idea.
- What would happen to the patient if the serum cholinesterase activity were only 10 units of activity per liter rather than the normal activity of about 80 units?
- Some patients have a mutant form of the serum cholinesterase that displays a KM of 10 mM, rather than the normal 1.4 mM. What will be the effect of this mutation on the patient?
- (a) This piece of information is necessary for determining the correct dosage of succinylcholine to administer.
(b) The duration of the paralysis depends on the ability of the serum cholinesterase to clear the drug. If there were one-eighth the amount of enzyme activity, paralysis could last eight times as long.
(c) KM is the concentration needed by the enzyme to reach ½ Vmax. Consequently, for a given concentration of substrate, the reaction catalyzed by the enzyme with the lower KMwill have the higher rate. The mutant patient with the higher KM will clear the drug at a much lower rate.
Question 8.33
KM matters. The amino acid asparagine is required by cancer cells to proliferate. Treating patients with the enzyme asparaginase is sometimes used as a chemotherapy treatment. Asparaginase hydrolyzes asparagine to aspartate and ammonia. The adjoining illustration shows the Michaelis–Menten curves for two asparaginases from different sources, as well as the concentration of asparagine in the environment (indicated by the arrow). Which enzyme would make a better chemotherapeutic agent?

- Enzyme 2. Despite the fact that enzyme 1 has a higher Vmax than enzyme 2, enzyme 2 shows greater activity at the concentration of the substrate in the environment because enzyme 2 has a lower KM for the substrate.
Question 8.35
Varying the enzyme. For a one-substrate, enzyme-catalyzed reaction, double-reciprocal plots were determined for three different enzyme concentrations. Which of the following three families of curve would you expect to be obtained? Explain.

- If the total amount of enzyme (ET) is increased, Vmax will increase, because Vmax = k2[E]T. But KM = (k−1 + k2)/k1; that is, it is independent of substrate concentration. The middle graph describes this situation.


Question 8.37
Too much of a good thing. A simple Michaelis–Menten enzyme, in the absence of any inhibitor, displayed the following kinetic behavior.
Draw a double-reciprocal plot that corresponds to the velocity-versus-substrate curve.
Suggest a plausible explanation for these kinetic results.


Question 8.38
Rate-limiting step. In the conversion of A into D in the following biochemical pathway, enzymes EA, EB, and EC have the KM values indicated under each enzyme. If all of the substrates and products are present at a concentration of 10−4 M and the enzymes have approximately the same Vmax, which step will be rate limiting and why?

- The first step will be the rate-limiting step. Enzymes EB and EC are operating at ½ Vmax, whereas the KM for enzyme EA is greater than the substrate concentration. EA would be operating at approximately 10−2 Vmax.



