Chapter 6: Diseases of the Immune System Part 2 Flashcards
What are the 3 immune-privileged sites?
- Brain
- Eyes
- Testis
How are antigens restricted to peripheral tissues able to be expressed in the thymus during the process of central tolerance?
A protein called AIRE
Mutations in the AIRE gene cause?
Autoimmune polyendocrinopathy
Function of CTLA-4 and PD-1?
- Structurally homologous to CD28 and act as inhibitory receptors
- CTLA-4 has a higher affinity for B7 molecules on APC’s
- Act to induce anergy in a lymphocyte upon recognizing a self antigen
What plays a role in acceptance/tolerance of a fetus while inside the placenta?
CD4+ CD25+ FOXP3 Treg cells
Which cytokine is essential for the maintenance of Treg cells?
IL-2
How are self-reactive T cells able to undergo apoptosis in the process of peripheral tolerance?
- Self-reactive T cells express pro-apoptotic member of Bcl family (Bim), w/o antiapoptotic members of the family like Bcl-2 or Bcl-x
- If self-antigens engage antigen receptors of self-reactive T cells, FasL and Fas are co-expressed inducing extrinsic pathways of apoptosis
Mutations in the FAS gene cause what disease?
Autoimmune Lymphoproliferative Syndrome (ALPS)
What HLA type is associated with Ankylosing spondylitis?
B-27 (mainly B*2705 and B*2702)
Which gene is said to be most frequently implicated in autoimmunity; encodes what?
Associated with what disorders?
- PTPN22; encodes a protein tyrosine phosphatase
- Type I DM, Rheumatoid arthritis, IBD
Polymorphisms in the gene for NOD2 are associated with what disease?
Chron’s disease
Polymorphisms in the genes encoding the IL-2R (CD25) and IL-7 receptor α chains are associated with what 2 diseases?
MS
Type I diabetes
What are 2 methods by which infections may induce autoimmunity?
1) Infections may upregulate the expression of co-stimulators on APCs; if these cells are presenting self-antigens result may be a breakdown of anergy and activation of self-reactive T cells
2) Molecular mimicry: microbe expresses Ag w/ same AA sequence as self-Ags
What is the classic example of molecular mimicry?
Causes what?
- Rheumatic heart disease; Abs against streptococcal proteins cross-react w/ myocardial proteins
- Cause Myocarditis
Some viruses like EBV and HIV cause __________which may result in the production of autoantibodies
Polyclonal B cell activation
How may infections actually protect against some autoimmune diseases?
Promoting low levels of IL-2 production which is essential for maintaining Treg cells
Most chronic inflammatory diseases are caused by abnormal and excessive?
TH1 and TH17 responses
*Psoriasis, MS, and some types of IBD
What group of people is most common to develop SLE?
- Women
- African American and Hispanic > White
What ANAs are specific for SLE?
- anti-dsDNA
- anti-Smith (Sm)
What antibodies are diagnostic for Sjogren syndrome?
- anti-Ro/SS-A
- anti-La/SS-B
Autoantibodies associated with Goodpasture Syndrome?
- anti-basement membrane
- anti-type IV collagen
Autoantibodies associated with drug-induced SLE?
anti-histone antibodies
Autoantibody specific for Rheumatoid Arthritis?
anti-CCP (cyclic citrullinated peptides)
Autoantibody associated with diffuse systemic scleroderma?
Limited systemic scleroderma?
- Diffuse = anti-DNA topoisomerase (anti-Scl 70)
- Limited (CREST) = anticentromere antibody
Injury in SLE is caused by?
What type of hypersensitivity?
- Deposition of immune complexes and binding of antibodies to various cells and tissues
- Type III hypersensitivity
Hallmark of SLE?
Production of autoantibodies
Patients with SLE that have the presence of antiphosphlipid antibodies will have increased?
These antibodies cause what kind of state?
- Increased PTT
- Hypercoagulable state (excessive clotting); leading to thrombosis
Specific alleles of which HLA has been linked to production of anti-dsDNA, anti-Sm, and anti-phospholipid antibodies seen in SLE?
HLA-DQ
What environmental factors are involved in SLE?
- UV light: may induce apoptosis in cells and alter DNA to become immunogenic
- Gender bias: partly related to genes on the X chromosome
- Drugs: such as hydralazine, procainamide, and D-penicillamine
What is the morphology of the blood vessels like in patients with SLE?
How about in the chronic stages?
- Acute necrotizing vasculitis involving capillaries, small arteries, and arterioles
- Chronic stages = vessels undergo fibrous thickening with luminal narrowing
What are the joints like in patients with SLE?
Opposite of what?
- Non-erosive synovitis with little deformity
- Opposite of RA
Glomerular lesions in SLE are the result of what?
Immune Complex Deposition
What is the most common and also the most severe pattern of glomerular disease seen in SLE?
Characteristics/morphology?
- Diffuse lupus nephritis (Class IV)
- >50% involvement of Glomeruli
- Proliferation of epithelial cells —> cellular crescents that fill Bowmans space
- Circumferential thickening of capillary wall, forming “wire loop” strucutres on light mircoscopy (see attached photo)

Venous and arterial thromboses, which may be associated with recurrent spontaneous miscarriages and focal cerebral or ocular ischemia is characteristic of?
Antiphospholipid antibody syndrome
- Can be primary = by itself
- Or secondary = in association with lupus
Immunofluorescence microscopy of the skin in patient with SLE will show?
Are these finding diagnostic?
- Deposition of immunoglobulin and complement along dermoepidermal junction
- May also be present in uninvolved skin
- This finding is NOT diagnostic of SLE and is sometimes seen in scleroderma or dermatomyositis

What kind of effusions may be present with SLE?
Pleural and pericardial effusions (sometimes with LE cells)
What cardiovascular system effects are present with SLE?
- Symptomatic or asymptomatic pericardial involvement (50% of pt’s)
- Myocarditis
- Valvular abnormalities (mitral and aortic)
- Valvular (Libman-Sack) endocarditis (see photo)
- CAD (angina, MI) owing to coronary atherosclerosis

What other organs are involved in SLE?
CNS
Spleen (onion-skin lesions from smooth muscle cell hyperplasia)
Lungs (effusions), interstitial fibrosis and secondary pulmonary HTN
What are signs of renal involvement in SLE?
Hematuria, proteinuria, red cell casts, nephrotic syndrome
What are the main clinical features of a patient presenting with SLE?
- Butterfly rash over the face
- Fever
- Pain but no deformity in one or more peripheral joints (feet, ankles, knees, hips, fingers, wrists, elbows, shoulders)
- Pleuritic chest pain
- Photosensitivity
What are the most common causes of death in patients with SLE?
- Renal failure
- Infections
- CAD
How does Chronic Discoid Lupus Erythematosus present?
- Skin manifestations mimicking SLE, rarely systemic involvement
- Skin plaques w/ edema, erythema, scaliness, follicular plugging
- Localized, deep, and scarring
- Usually only involving the face and scalp
How is Subacute Cutaneous Lupus distinguished from Chronic Discoid LE?
- Skin rash is widespread, superficial, and non-scarring
- Most have mild systemic symptoms consistent with SLE
- Strong association with antibodies to the SS-A antigen and w/ HLA-DR3 genotype
*Basically an intermeditate between SLE and Chronic Discoid LE
What drugs induce lupus syndrome?
Hydralazine
Procainamide
Isoniazid
D-penicillamine
*Anti-TNF used to tx RA, may also induce lupus*
What organs are NOT affected in drug-induced LE?
Renal
CNS
What is Sjorgren characterized by?
Result of?
- Dry eyes (keratoconjunctivitis sicca) and dry mouth (xerostomia)
- Immunologically mediated destruction of lacrimal and salivary glands
Sjorgen Syndrome can occurs as a primary form (sicca syndrome) or most commonly as a secondary form, which disease is it most commonly associated with?
- Rheumatoid arthritis
- Can also be associated with SLE, polymyositis, scleroderma, vasculitis, mixed CT disease, or thyroiditis
Which autoantibody found in patients with Sjorgen syndrome is associated with early disease onset, longer disease duration, and extraglandular manifestations, such as diffuse pulmonary fibrosis, cutaneous vasculitis and nephritis?
SS-A
What is the earliest histological finding in both the major and minor salivary glands in patients with Sjorgen Syndrome?
Periductal and perivascular lymphocytic infiltration
Patients with Sjorgen Syndrome are at a high risk for developing?
Dominant B-cell clone and marginal zone lymphoma
What are the clinical features and presenting signs of a patient presenting with Sjorgen Syndrome?
Most common gender and age group affected?
- Women between ages 50-60
- Keratoconjunctivitis = Blurring of vision, burning, and itching, and thick secretions accumulate in the conjunctival sac
- Xerostomia = Difficulty swallowing solids, decreased taste, cracks and fissures in mouth
- Parotid gland enlargement
- Epistaxis
- Recurrent bronchitis and pneumonia
What is essential for the diagnosis of Sjorgen Syndrome?
Biopsy of the lip to examine minor salivary glands
What sort of renal involvement is seen in Sjorgen Syndrome vs. SLE?
- Defects of tubular function (renal tubular acidosis, uricosuria, and phosphaturia)
- Glomerular lesions are extremely rare (like those seen in SLE)
Systemic Sclerosis (Scleroderma) is characterized by what 3 things?
1) Chronic inflammation though to be result of autoimmunity
2) Widespread damage to small blood vessels
3) Progressive interstitial and perivascular fibrosis in the skin (early) and multiple organs (later)
Systemic Sclerosis (Scleroderma) is characterized by excessive?
Fibrosis throughout the body
Which organs are most commonly involved in Systemic Sclerosis (Scleroderma)?
- Skin is most common
- GI tract
- Kidneys
- Heart
- Muscles
- Lungs
Majority of patients with Systemic Sclerosis (Scleroderma) die how?
- Renal failure
- Cardiac failure
- Pulmonary insufficiency
- Intestinal malabsorption
Some patients with the limited form of Systemic Sclerosis (Scleroderma) also develop?
- CREST syndrome
- Calcinosis
- Raynaud phenomenon
- Esophageal dysmotility
- Sclerodactyly
- Telangiectasia
What are the Clinical features of Systemic Sclerosis (Scleroderma)?
Who’s affected most often (gender and age)?
- Female-to-male ratio of 3:1
- More severe in blacks
- Peak incidence in the 50-60 yo group
- Distinctive features = cutaneous changes, skin thickening, Raynaud phenomenon
- Dysphagia, respiratory difficulty, myocardial fibrosis, mild proteinuria
What is the major cause of death in patients with Systemic Sclerosis (Scleroderma)?
Pulmonary disease
What leads to the fibrosis in Systemic Scleroderma?
Activation of fibroblasts by cytokines produced by T cells and alternatively activated macrophages (M2)
Mixed Connective Tissue disease is characterized serologically by high titers if antibodies to?
Ribonucleotide particle-containing U1 ribonucleoprotein (anti-U1-ribonucleotide)
How does Mixed CT disease typically present?
Good response to what tx?
- Synovitis of the finger
- Raynaud phenomenon and mild myositis
- Renal involvement is modest
- Good response to corticosterioid, in the short term
What disease often affects middle-aged and older men and is 1st characterized in autoimmune pancreatitis?
IgG4 Related Disease
What is Mikulicz syndrome?
Seen in what conditions?
- Enlargement and fibrosis of salivary and lacrimal glands
- Sjorgen syndrome and IgG4 related disease
What is IgG4 related disease characterized by?
- Tissue infiltrates dominated by IgG4 anti-body producing plasma cells and lymphocytes (mainly T cells)
- Storiform fibrosis
- Obliterative phlebitis
- Increased serum IgG4
What is the direct pathway for recognition of alloantigens in organ grafts?
Why are the DCs in the donor organs most important for initiating the antigraft response?
- T cells of recipient recognize allogenic (donor) MHC molecules on the surface of donor APCs in the graft
- The APCs of the donor organ express high levels of class I and class II MHC molecules and are also endowed with costimulatory molecules (B7)

What is involved in the indirect pathway of allorecognition in organ grafts?
Which immune cells are involved here?
- Peptides derived from the donor tissue are presented by the host’s own MHC molecules, like any other foreign peptide
- CD4+ T cells enter the graft and recognize graft antigens being displayed by host APCs that have also entered the graft, resulting in delayed hypersensitivity type of inflammation.
- CD4+ T cells also stimulate B lymphocytes —> plasma cells to produce antibodies and also macrophages
- CD8+ CTLs may be generated via this pathways, but cannot kill graft cells, because these CTLs recognize graft antigens presented by host APCs and cannot recognize the graft cells directly

What kind of damage occurs from CD8+ CTLs that may be generated from the indirect pathway for recognition of alloantigens in organ grafts?
What is the principle mechanism of cellular rejection in the indirect pathway?
- CD8+ CTLs CANNOT kill graft cells becauses these CTLs recognize graft antigens presented by the host APCs and cannot recognize the graft cells directly
- Principle mechanism of cellular injury = T-cell cytokine production and inflammation

What characterizes acute cellular rejection; cell type responsible?
Graft injury is caused by?
Commonly seen when?
- CD4+ T cells secrete cytokines causing inflammation characterized by increased vascular permeability and local accumulation of mononuclear cells (lymphocytes and macrophages)
- Graft injury is caused by activated macrophages
- Occurs within initial months following transplantation

What characterizes chronic cellular rejection of a transplanted organ?
Patients may present clinically with progressive renal failure manifested by a rise in serum levels of?
- Lymphocytes reacting against alloantigens in the vessel wall secrete cytokines that induce local inflammation
- May stimulate proliferation of vascular endothelial and smooth muscle cells
- Rise in serum creatinine over period of 4-6 months
What causes hyperacute rejection of an donor organ?
Most commonly seen in what circumstances?
How fast is the rejection?
- Occurs when preformed antidonor antibodies are present in circulation of reciepient
- Can be caused by: previous transplant, prior blood transfusions, and multiparous women
- Occurs within minutes to hours

Fibrinoid necrosis ans thrombosis are typical of what kind of rejection reaction?
Hyperacute rejection

In acute antibody-mediated rejection what seems to be the target of the antibodies formed?
How quick does this rejection reaction occur?
- Graft vasculature
- May occur within days or may suddenely appear months or even years later after immunosuppression is tapered or terminated

How is chronic antibody-mediated rejection different from acute?
Primarily affects what part of grat?
- Occurs slower (more insidiously); without preceding acute rejection
- Antibodies are detected in circulation, but are NOT readily identified within the graft
- Primarily affects graft vasculature
Acute cellular (T cell-mediated) rejection may be seen as 2 patterns histologically, what are they?
- Tuberointerstitial pattern (type I): extensive interstitial inflammation w/ infiltration of tubules, called tubulitis
- Vascular pattern shows inflammation of vessels (endothelitis, type II), sometimes with necrosis of vascular walls (type III)

Lesions consisting of inflammation of glomeruli and pertibulular capillaries, associated with deposition of the complement breakdown product C4d is associated with what type of rejection reaction?
Acute antibody-mediated rejection

Chronically rejecting kidneys usually have interstitial mononuclear cell infiltrates, including what cells?
NK cells
Plasma cells
What alleles are matched for kidney transplants?
Both inherited alleles of HLA-A, HLA-B, and HLA-DR
What type of transplants is allele matching not done for?
Liver, Heart, Lungs
What 3 drugs are given for immunosuppressive therapy?
Basic function of each?
- Steroids (reduce inflammation)
- Mycophenolate mofetil (inhibits lymphocyte proliferation)
- Tacrolimus (FK506) (inhibits T cell functions)
What is the MOA for Tacrolimus given as an immunosuppressant?
- Inhibitor of phosphatase calcineurin, which is required for activation of NFAT
- NFAT stimulates transcription of gene encoding IL-2
- Thus, Tacrolimus inhibits T cell functions
What can be given as an immunosuppressant that is a suppressor of inflammation by unknown mechanisms?
What is used in cases of severe antibody-mediated rejection?
- Pooled intravenous IgG (IVIG)
- Plasmapheresis in severe cases
In kidney transplant recipients what virus can reactivate and infect renal tubules, may even cause graft failure?
How to stop?
- Polyoma Virus
- Reduce immunosuppression
Immunosuppressed individuals such as those receiving organ transplants are at increased risk for developing what from the reactivation of latent viruses?
- EBV-induced lymphomas
- HPV-induced squamous cell carcinomas
- Kaposi sarcoma (HHV8)
What is long-term mixed chimerism in regards to graft recipients and why may this be an effective strategy for reducing reactions to grafts?
- Giving donor cells to graft recipients may prevent rxns to the graft, because the donor inoculum contains cells, such as immature DCs, that induce tolerance to the donor allo-antigens
- Long-term mixed chimerism is when a recipient lives with the injected donor cells
What conditions is the transplantation of Hematopoietic Stem Cells used for?
- Hematologic malignancies
- Bone marrow failure syndrome (such as aplastic anemia)
- Inherited stem cell defects (such as sickle cell anemia, thalassemia, and immunodeficiency states)
When is GVHD most commonly seen in the setting of?
HSC transplantation
Patient presents with a rash and diarrhea after weeks of allogenic bone marrow transplantation, what is going on?
Acute GVHD
What are the major organs affected from acute GVHD?
Immune system, skin, liver, and intestines
Patient present with extensive cutaneous injury, with destruction of skin appendages and fibrosis of the dermins after a bone marrow transplant, what type of reaction is this?
Chronic GVHD
What characterizes chronic GVHD?
- Extensive cutaneous injury
- Chronic liver disease manifested by cholestatic jaundice –> Hyperbilirubinemia
What mediates GVHD?
How can this disease be eliminated before transplantation?
- Mediated by T lymphocytes contained in the donor cells
- Depletion of donor cells before transfusion virtually eliminates the disease
Depletion of the donor T cells before transfusion of hematopoietic stem cells virtually eliminates GVHD, but unfortunately can lead to what issues?
- Recurrence of tumor in leukemia patients
- Increased incidence of graft failure
- Increased rates of EBV-related B-cell lymphoma
What is the graft-versus-leukemia effect used to treat?
Deliberate induction of graft-versus-leukemia effect by infusion of allogenic T cells is used to treat myelogenous leukemia that has relapsed after HSC transplantation
Immunodeficient individuals who received an HSC transplantation are at risk for many infections, but which is particularly important?
What can be a fatal complication of this virus?
- Cytomegalovirus
- Cytomegalovirus-induced pneumonitis
What is the defect in leukocyte adhesion deficiency type 1 and type 2?
Type 1 = defect in biosynthesis of the β2 chain shared by LFA-1 and Mac-1 integrins
Type 2 = absence of sialyl-Lewis X, fucose containing ligand for E- and P-selectins
What is the inheritance pattern of Chediak-Higashi syndrome?
Autosomal Recessive
Chediak-Higashi syndrome is characterized by?
Gene defect encoding which cytosolic protein?
- Defective fusion of phagosomes and lysosome, resulting in defective phagocytes function and susceptibility to infections
- Neutropenia, abnormalities in melanocytes (leading to albinism), cells of NS (nerve defects), and platelets (causing bleeding disorders)
- Gene defect encodes cytosolic protein LYST (regulates lysosomal trafficking)
Chronic granulomatous disease is characterized by?
- Defects in bacterial killing
- Inherited defect in genes encoding components of phagocyte oxidase, phagolysosomal enzyme that generates superoxide (O2-*)
SCID affects what immune responses?
Defects in both Humoral and Cell-mediated immune response
Infants with SCID present how?
- Prominent thrush (oral candidiasis)
- Extensive diaper rash
- Failure to thrive
Persons with SCID are extremely susceptible to which recurrent, severe infections? (mnemonic)
PPV w/ ur CC
Pneumocystis jiroveci
Psuedomonas
Varicella
Candida albicans
Cytomegalovirus
Without __________, death occurs within the first year of life in patients with SCID
HSC transplantation
What is the most common inheritance pattern for SCID?
What is the mutation caused by this form?
- X-linked is most common (more common in boys than girls)
- Mutation in the common γ-chain (γc) subunit of cytokine receptors
Which cytokine affected by the mutation in the X-linked form of SCID is necessary for the survival and proliferation of lymphoid progenitors, particularly T cell precursors?
Which cytokine is important for the maturation and proliferation of NK cells and is also affected by this mutation?
- IL-7 (lymphoid progenitors)
- IL-15 (NK cells)
The most common cause of autosomal recessive SCID is a deficiency in what enzyme?
Adenosine deaminase (ADA)
X-linked Agammaglobulinemia is characterized by failure of what?
Caused by mutations in?
- Failure of B-cell precursors (pro-B cells and pre-B cells) to develop into mature B cells
- Mutations in a cytoplasmic tyrosine kiase, called Bruton tyrosine kinase (Btk)
- Gene encoding this enzyme is located on long arm of the X chromosome at Xq21.22
Patients affected by X-linked Agammaglobulinemia usually do not shows signs until what age?
About 6 months of age, as maternal immunoglobulins are depleted
What is the first human disease in which gene therapy has been successful?
X-linked SCID
Patients with X-linked Agammaglobulinemia at increased risk for which recurrent infections?
Caused by what organisms?
- Recurrent bacterial infections of the respiratory tract, such as acute and chronic pharyngitis, sinusitis otitis media, bronchitis, and pneumonia
- Haemophilus influenzae, Streptococcus pneumoniae, or Staphylococcus aureus
The classic form of X-linked Agammaglobulinemia has what characteristics (i.e., immune cells throughout the body)?
- B cells are absent or markedly decreased
- Serum levels of all classed of immunoglobulins are depressed
- Plasma cells are absent
- T-cell mediated rxns are normal
What viruses are common in X-linked Agammaglobulinemia?
Poliovirus
Enterovirus (echovirus)
Coxsackievirus
Giardia lamblia
*PECG*
35% people with X-linked Agammaglobulinemia develop what?
Autoimmune diseases, such as arthritis and dermatomyositis
What is the treatment for X-linked Agammaglobulinemia?
Replacement therapy with immunoglobulins
DiGeorge Syndrome is due to what?
Deficiency in what?
What chromosome abnormality?
- Failure of 3rd and 4th pharyngeal pouches
- T-cells
- 22q11 deletion
What characterizes Hyper-IgM syndrome?
Defect in what?
- Patients makes IgM, but deficient in IgA, IgG, and IgE
- Defect in ability of helper T cells to deliver activating signals to B cells and macrophages (defect in CD40L or CD40)
Sx of DiGeorge Syndrome?
- Poor defense against fungal and viral infections
- Lack of parathyroids –> Hypocalcemia –> tetany
- Congenital defects of heart and great vessels
- Abnormal facial and ear appearance
What kind of inheritance with Hyper-IgM syndrome?
What is the defect in each of these inheritance patterns?
- X-linked = most common = defect in gene encoding CD40L (on T helper cells)
- Autosomal recessive = mutation in CD40 or AID (DNA editing enzyme required for Ig class swithching/affinity maturation)
Patients with Hyper-IgM syndrome present with what type of recurrent infections?
Due to?
Those with the CD40L mutations also susceptible to?
- Recurrent pyrogenic infections
- Due to low level of opsonizing IgG
- CD40L mutations also susceptible to pneumonia caused by Pneumocystis jiroveci
Common Variable Imunnodeficiency in contrast to X-linked agammaglobulinemia is characterized by what?
- Near-normal number of B cells in the blood and lymphoid tissues; however these B cell are not able to differentiate into plasma cells
- Affects both sexes EQUALLY
- Onset of sx’s is later, in childhood or adolescence
Isolated IgA deficiency is common in whom?
Susceptible to what problems?
- European descent (1 in 600)
- Mucosal defenses weakened, and infections occur in respiratory, GI, and urogenital tracts
- Symptomatic pt’s commonly present w/ recurrent sinopulmonary infections and diarrhea
What problem can occur if someone needs a blood transfusion and is asymptomatic with Isolated IgA deficiency?
- If transfused with blood containing normal IgA, some pt’s can develop severe, even fatal, anaphylactic rxns
- The IgA behaves like a foreign antigen (since the pt’s do not produce or tolerate it)
X-linked Lymphoproliferative Syndrome is characterized by an inability to eliminate?
Eventually leads to?
- Inability to eliminate Epstein-Barr virus (EBV)
- Eventually leading to fulminant infections mononucleosis and development of B-cell tumors
X-linked Lymphoproliferative Syndrome is due to mutations in the gene encoding which adaptor molecule?
What is the function of this molecule?
- SLAM-associated protein (SAP)
- Binds to family of cell surface molecules invovled in activation of NK cells and T and B lymphocytes, includin signaling lymphocyte activation molecule (SLAM)
Cause of chronic mucocutaneous candidiasis as well as bacterial infections of the skin (called Job syndrome)?
Defective TH17 responses
X-linked disease characterized by thrombocytopenia, eczema, and a marked vulnerability to recurrent infection, resulting in early death?
Wiskott-Aldrich Syndrome
Wiskott-Aldrich Syndrome is characterized by what levels of immunoglobulins?
- IgM is low
- IgG is normal
- IgA and IgE elevated
What is the inheritance pattern of Ataxia Telangiectasia?
Characterized by?
- Autosomal Recessive
- Abnormal gait (ataxia), vascular malformations (telangiectases), neurologic deficits, increased incidence of tumors, and immunodeficiency
Most prominent humoral immune abnormalities in Ataxia Telangiectasia are defective production on?
Isotype switching antibodies, mainly IgA and IgG2
Gene responsible for Ataxia Telangiectasia is located on what chromosome?
Encodes what protein?
- Chromosome 11
- Encodes protein called ATM that is structurally related to (PI-3) kinase
- ATM protein is a sensor of DNA damage (double strand breaks) and activates p53 by phosphorylation
What can cause secondary immunodeficiency? (mnemonic)
Malnutrition
Chemotherapy
Irradiation
Cancers
Infections
My Cat Is Catching Insects
Globally what is the most common mode of transmission of HIV?
Heterosexual contacts
Two genetically different but related forms of HIV (HIV-1 and HIV-2) have been isolated from patients with AIDS, where is each most commonly found?
HIV-1 = most commonly seen in United States, Europe, and Central Africa
HIV-2 = West Africa and India
What major capsid protein is the most abundant viral antigen and what test is used to diagnose HIV infection?
- p24
- ELISA
What is contained in the core of the HIV?
- Major capsid protein p24
- Nucleocapsid protein p7/p9
- 2 copies of viral genomic RNA
- 3 viral enzymes: protease, reverse transcriptase, and integrase
What is the virus core of HIV surrounded by?
Matrix protein p17
What studs the viral envelope of HIV?
gp120 and gp41
What does HIV first infect?
What do they all have?
- T cells, DCs, and macrophages
- All have CD4
Which type of HIV is the dominant virus found in the blood of acutely infected individuals and early in the course of the infection?
Over the course of the infection which type gradually accumulates and are especially virulent?
- R5 (M-tropic) type
- T-tropic accumulate and are especially virulent
The M-tropic strain preferentially infects what?
What strain and chemokine receptor?
- Monocyte/macrophages
- R5 strain uses CCR5
What is the intial steps in infection by HIV (binding of)?
Binding of coreceptor induces what change and effect?
- Binding of gp120 to CD4, leads to conformation change resulting in formation of new recognition site on gp120 for the coreceptors CC5 or CXCR4
- Binding of coreceptors induces conformational changes in gp41 resulting in exposure of a hydrophobic region called the fusion peptide at the tip of gp41
- Peptide inserts into the cell membrane of the target cells, leading to fusion of the virus and the host cell

The T-tropic strain uses what strain and chemokine receptor?
X4 and CXCR4
Which HIV viral envelope glycoprotein is able to penetrate the target cell membrane?
gp41

Which T cells are infected by HIV?
- Memory and activated T cells
- HIV is inefficient at infecting naive (unactivated) T cells
Which enzyme is active in naive T cells that introduces mutations in the HIV genome?
HIV has evolved to counteract this cellular defense mechanism by using?
- APOBEC3G
- Vif
Activation of T cells leads to upregulation of what transcription factor?
How does HIV use this transcritption factor?
- NF-kB
- HIV genome contains NF-kB binding sites, and induction of NF-kB activates the transcription of HIV proviral DNA
HIV-1 can infect and multiply in terminally differentiated nondividing macrophages, and is dependent on what viral gene?
Vpr gene
Although macrophages allow viral replication, they are quite resistant to the cytopathic effects of HIV, in contrast to CD4+ T cell. Instead macrophages act as?
- Reservoirs of infection, especially when CD4+ T cell numbers are declining, macrophages allow for continued viral replicaton
- Act as portals of infection; early in the course of the infection and the acute phase the M-tropic strains are the predominant circulating form
What is the role Mucosal DCs and Follicular DCs in HIV infection?
Mucosal DCs are infected by the virus and may transport it to regional LNs
Follicular DCs in the germinal centers of LNS are potential reservoirs of HIV
What are the predominate cell types in the brain infected by HIV?
Macrophages and Microglial cell
HIV-infected macrophages produce increased amounts of what cytokine; which causes?
HIV-infected macrophages –> IL-6 —> B-cell proliferation
Acute retroviral syndrome occurs when?
What are the symptoms?
- Occurs 3-6 weeks after infection and resolves spontaneously after 2-4 weeks
- “Flu-like symptoms”: sore throat, myalgias, fever, weight loss, and fatigue
- Rash, cervical adenopathy, diarrhea, vomiting

For clinical managment of HIV, blood levels of what are the most reliable short-term indicator of disease progression?
CD4+ T-cell counts
Where are the sites of continuous HIV replication and cell destruction during the clinical latency period?
Lymph nodes
Spleen
Viral load at the end of the acute phase of HIV infection reflects which equilibrium?
How is this related to the prognosis?
- Equilibrium reached between the virus and host response
- Level of steady state viremia, called the viral set point, is a predictor of the rate of decline of CD4+ T cells and progression of HIV disease
How does HIV evade immune detection?
- Destroys CD4+ T cells critical for effective immunity
- Antigenic variation
- Down-modulates class I MHC on infected cells so not recognized by CD8+ CTLs
- Evovles and switches from relying solely on CCR5 to enter target cells, can now use both CCR5 and CXCR4
In the chronic phase of HIV infection, patients are either asymptomatic, or develop minor opportunistic infections such as?
- Oral candidiasis (thrush)
- Vaginal candidiasis
- Herpes Zoster
- Mycobacterial tuberculosis (common in resource-poor regions - sub-Sahara)
What accounts for the majority of deaths in patients with untreated AIDS?
Opportunistic infections
Most common fungal infection in patients with AIDS?
Candidiasis
Cytomegalovirus retinitis occurs almost exclusively in patients with?
CD4+ T cell counts less than 50 per microliter
Immune reconstitution inflammatory syndrome may occur with what?
HAART treatment given to some patients with advanced disease
Patients with AIDS have a high incidence of which tumors?
- Kaposi sarcoma (KS)
- B-cell lymphoma
- Cervical cancer (woman)
- Anal cancer (men)
Most common neoplasm in patients with AIDS?
Caused by what virus
- Kaposi Sarcoma, a vascular tumor
- Human herpes virus 8 (HHV8)
Amyloidosis is caused by extracellular deposits of?
Misfolded fibrillar proteins which aggregate and lead to tissue damage and functional compromise
What are the adverse side effects of HAART?
- Lipoatrophy (loss of facial fat)
- Lipoaccumulation (excess fat deposition centrally)
- Elevated lipids
- Insulin resistance
- Peripheral neuropathy
- Premature cardiovascular, kidney, and liver disease
Which histochemical method is most widely used to differentiate amyloid from other hyaline materials?
What molecular feature causes the reaction?
- Congo red stain, which under ordinary light imparts a pink or red color to tissue deposits, but far more striking is the green birefringence of the stained amyloid when oberved by polarizing microscopy
- Caused by the β-pleated configuration
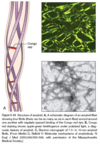
What is AL (amyloid light chain) protein made up of?
When analyzed are composed of?
- Made up of complete immunoglobulin light chains
- When analyzed composed of λ light chains or their fragments, but K light chains present sometimes too
The AA (amyloid-assocatied) type of amyloid fibril protein is derived from?
When is production of the precursor molecule increased?
- Proteolysis of a larger precursor in the serum called SAA, that is synthesized in the liver
- SAA protein is increased in inflammatory states as part of the acute phase response, therefore, this form of amyloidosis is associated with chronic inflammation
β-amyloid protein (Aβ) is seen in what condition?
Derived from what precursor?
- Core of cerebral plaques seen in Alzheimers
- Dervided from proteolysis of larger glycoprotein, called amyoid precursor protein (APP)
What is found deposited in the heart of older individuals and contributes to senile systemic amyloidosis?
TTR
Hemodialysis associated Amyloidosis is from what major fibril protein?
What precursor protein?
What associated disease?
- Aβ2m
- β2-microglobulin (pre-cursor)
- Chronic renal failure
Immunocyte dyscrasias with amyloidosis (primary amyloidosis) has which major fibril protein?
Chemically related precursor protein?
Associated diseases?
- AL = most common form of amyloidosis
- Ig light chains, chiefly λ type
- Multiple myeloma and other monoclonal plasma cell proliferations
What is a Bence-Jones protein?
Found where?
- Malignant plasma cells often secrete free, unpaired κ or λ light chain
- Found in the serum and excreted/concentrated in the urine
More commonly now reactive systemic amyloidosis complicates which conditions?
- Rheumatoid arthritis
- Ankylosis spondylitis
- IBD
- Chron disease and Ulcerative colitis
Reactive systemic Amyloidosis has what major fibril protein associated with it?
What precursor protein?
What associated disease?
- AA
- SAA
- Chronic inflammatory condition
Patients with Hemodialysis-Associated Amyloidosis sometimes present with?
Carpal Tunnel Syndrom because of β2-microglobulin deposition
Familial Mediterranean fever is inherited how?
Defective gene encodes?
Seen in which populations?
Which type of amyloid seen in this condition?
- Autosomal recessive
- Defective gene encodes = Pyrin
- Armenian, Sephardic Jewish, and Arabic origins
- AA proteins
Type 1 Diabetes and M.S. Are what kind of diseases?
Organ Specific
In medullary carcinomas of the thyroid, the presence of ______ is an essential diagnostic feature.
Amyloid
Histologically, amyloid deposition is always?
Begins where?
Always extracellular and begins between cells
Amyloidosis of which organ is most common and also the most serious form of organ involvement?
Kidney
What is shown here?

Amyloidosis of the Liver

What is shown here?

Amyloidosis of the kidney

Which test is quite specific, but has low sensitivity for the diagnosis of systemic amyloidosis?
Examination of abdominal fat aspirates with Congo red
In some cases AL amyloid binds and inactivates ________, leading to a life-threatening bleeding disorder.
Factor X


